Caffeine
From Proteopedia
| Line 13: | Line 13: | ||
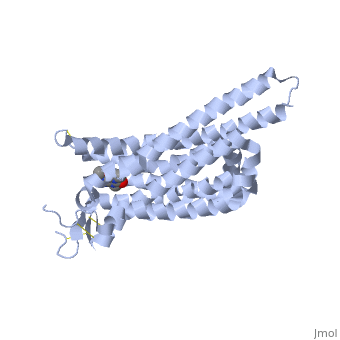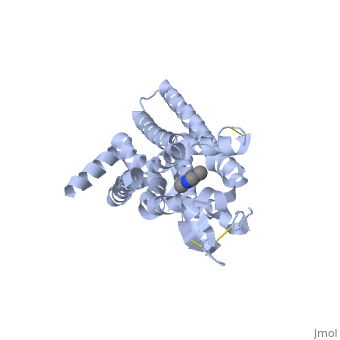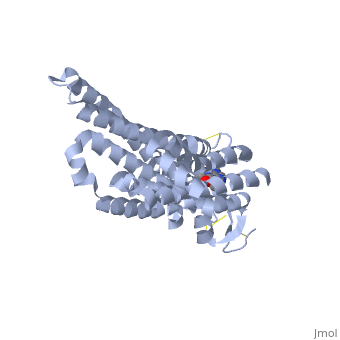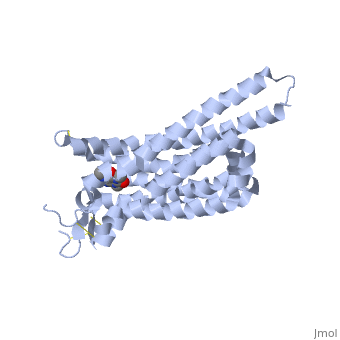
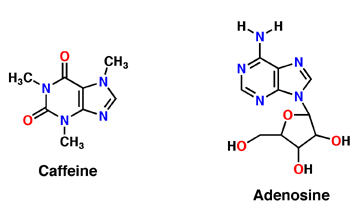
Adenosine is an inhibitory neurotransmitter, which promotes sleep and inhibits arousal. It has two components; an adenine nucleotide and a ribose sugar. Adenosine is a polar molecule and is water soluble. Within the brain, concentration of this neuromodulator increases every hour. Adenosine binds extracellularly to G-protein and induces multiple effects. The G-protein is composed of 7 alpha helices, which provide its secondary structure, and is a transmembrane protein. As adenosine receptors bind G-protein, neural activity begins to decrease and the person feels fatigued and sleepy. A2A receptor is one of many adenosine G protein-coupled receptors. | Adenosine is an inhibitory neurotransmitter, which promotes sleep and inhibits arousal. It has two components; an adenine nucleotide and a ribose sugar. Adenosine is a polar molecule and is water soluble. Within the brain, concentration of this neuromodulator increases every hour. Adenosine binds extracellularly to G-protein and induces multiple effects. The G-protein is composed of 7 alpha helices, which provide its secondary structure, and is a transmembrane protein. As adenosine receptors bind G-protein, neural activity begins to decrease and the person feels fatigued and sleepy. A2A receptor is one of many adenosine G protein-coupled receptors. | ||
| - | <Structure load='4UHR' size='250' frame='true' align=' | + | <Structure load='4UHR' size='250' frame='true' align='left' caption='Agonist Bound to A2A Receptor' scene='' /> |
| Line 30: | Line 30: | ||
== Trimethylxanthine == | == Trimethylxanthine == | ||
| - | A2A is a transmembrane G protein in humans. Trimethylxanthine has a highly water soluble and thus when present in the system, interacts with the A2A receptor. In order for Trimethylxantine to bind to the receptor, the third and seventh transmembrane helical domains need to recognize the ligand. Trimethylxanthine can then bind. Trimethylxanthine can bind with very little discomfort, due to its similar structure, as well as its purine alkaloid structure, to adenosine. This binding will change the shape and not initiate the cascade of downstream effects that adenosine does, like opening of ion channels and slowing of activity. Concentrate of free adenosine increases extracellularly, when trimethylxanthine is bound. The cAMP increases when adenosine is bound | + | A2A is a transmembrane G protein in humans. Trimethylxanthine has a highly water soluble and thus when present in the system, interacts with the A2A receptor. In order for Trimethylxantine to bind to the receptor, the third and seventh transmembrane helical domains need to recognize the ligand. Trimethylxanthine can then bind. Trimethylxanthine can bind with very little discomfort, due to its similar structure, as well as its purine alkaloid structure, to adenosine. This binding will change the shape and not initiate the cascade of downstream effects that adenosine does, like opening of ion channels and slowing of activity. Concentrate of free adenosine increases extracellularly, when trimethylxanthine is bound. The cAMP increases when adenosine is bound. ERK1 and ERK2 are kinases, composed of serine and threonine, of the GMGC group that regulation of cell growth and differentiation, and if adenosine was bound, this cascade of events would occur, but when Trimethylxanthine is bound, this regulation does not occur. |
== See Also == | == See Also == | ||
Revision as of 03:55, 17 November 2015
Caffeine
| |||||||||||
References
Denoeud, F., L. Carretero-Paulet, A. Dereeper, G. Droc, R. Guyot, M. Pietrella, C. Zheng, A. Alberti, F. Anthony, G. Aprea, J.-M. Aury, P. Bento, M. Bernard, S. Bocs, C. Campa, A. Cenci, M.-C. Combes, D. Crouzillat, C. Da Silva, L. Daddiego, F. De Bellis, S. Dussert, O. Garsmeur, T. Gayraud, V. Guignon, K. Jahn, V. Jamilloux, T. Joet, K. Labadie, T. Lan, J. Leclercq, M. Lepelley, T. Leroy, L.-T. Li, P. Librado, L. Lopez, A. Munoz, B. Noel, A. Pallavicini, G. Perrotta, V. Poncet, D. Pot, Priyono, M. Rigoreau, M. Rouard, J. Rozas, C. Tranchant-Dubreuil, R. Vanburen, Q. Zhang, A. C. Andrade, X. Argout, B. Bertrand, A. De Kochko, G. Graziosi, R. J. Henry, Jayarama, R. Ming, C. Nagai, S. Rounsley, D. Sankoff, G. Giuliano, V. A. Albert, P. Wincker, and P. Lashermes. "The Coffee Genome Provides Insight into the Convergent Evolution of Caffeine Biosynthesis." Science 345.6201 (2014): 1181-184.
Proteopedia Page Contributors and Editors (what is this?)
Mackenzie Kincer, Sidiqullah Halim, Alexander Berchansky, Michal Harel