Caffeine
From Proteopedia
| Line 1: | Line 1: | ||
| - | ==Caffeine (Trimethylxanthine) == | + | == The Affect of Caffeine (Trimethylxanthine) on Human A2A Receptor == |
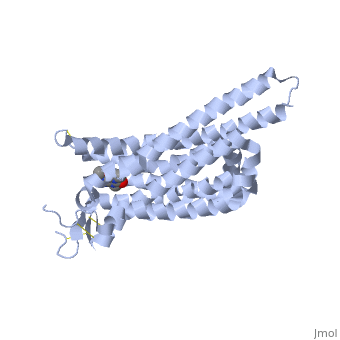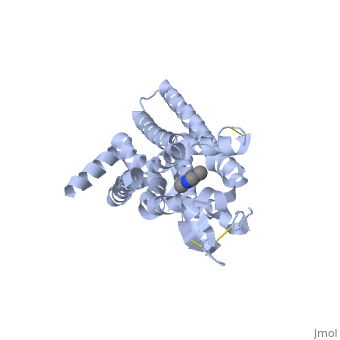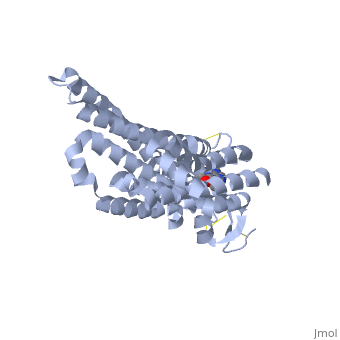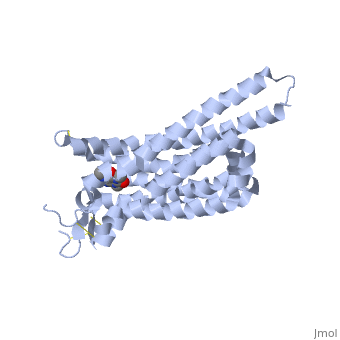
<StructureSection load='3RFM' size='340' side='right' caption='Caffeine Bound to A2A Receptor' scene=''> | <StructureSection load='3RFM' size='340' side='right' caption='Caffeine Bound to A2A Receptor' scene=''> | ||
| Line 9: | Line 9: | ||
== Caffeine (Trimethylxanthine) == | == Caffeine (Trimethylxanthine) == | ||
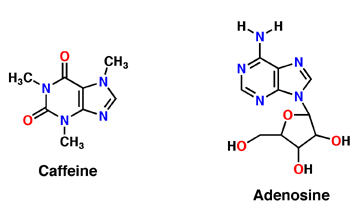
| - | Caffeine is a derivative of adenosine and is also called Trimethylxanthine. It is composed of purines; structurally it is polar, and water soluble. They antagonize or inhibit many of the adenosine receptors, like the A1 receptor mentioned above. Caffeine affects neurons and glial cells in the brain by binding to the same location that adenosine would bind and then induce a cascade of enzymatic downstream effects. | + | Caffeine is a derivative of adenosine and is also called Trimethylxanthine. It is composed of purines; structurally it is polar, and water soluble. They antagonize or inhibit many of the adenosine receptors, like the A1 receptor mentioned above. Caffeine affects neurons and glial cells in the brain by binding to the same location that adenosine would bind and then induce a cascade of enzymatic downstream effects (Denoeud ''et al'' 2014). |
<scene name='71/716555/Caffeine_binding/1'>A2A Receptor with Caffeine Bound</scene> | <scene name='71/716555/Caffeine_binding/1'>A2A Receptor with Caffeine Bound</scene> | ||
| Line 16: | Line 16: | ||
== Mechanism of Caffeine (Trimethylxanthine) Synthesis == | == Mechanism of Caffeine (Trimethylxanthine) Synthesis == | ||
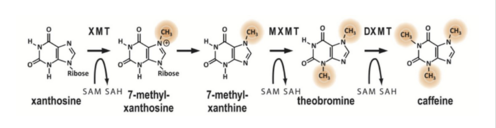
| - | Caffeine is a naturally occurring methylxanthine, purine alkaloid, synthesized by eudicot plants such as coffee, cacao, and tea (Denoeud et. al, 2014). In order to synthesize caffeine, xanthosine must undergo 3 methylation steps with the help of three NMT enzymes; xanthosine methyltransferase (XMT), theobromine synthase (MXMT), and caffeine synthase (DXMT) (Denoeud et. al, 2014). The first step of caffeine biosynthesis involves XMT converting S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) which removes a methyl group and adds it to the 7’-Nitrogen. This produces the intermediate 7-methyl-xanthosine to become 7-methyl-xanthine (Denoeud et | + | Caffeine is a naturally occurring methylxanthine, purine alkaloid, synthesized by eudicot plants such as coffee, cacao, and tea (Denoeud et. al, 2014). In order to synthesize caffeine, xanthosine must undergo 3 methylation steps with the help of three NMT enzymes; xanthosine methyltransferase (XMT), theobromine synthase (MXMT), and caffeine synthase (DXMT) (Denoeud et. al, 2014). The first step of caffeine biosynthesis involves XMT converting S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) which removes a methyl group and adds it to the 7’-Nitrogen. This produces the intermediate 7-methyl-xanthosine to become 7-methyl-xanthine (Denoeud ''et al'' 2014). The second enzyme, MXMT, converts another SAM to SAH, subsequently add a methyl group to the 3’- Nitrogen on 7-methyl-xanthine. This produces theobromine which may undergo another methylation step with the help of the enzyme DXMT. DXMT converts a third SAM to SAH, adding a methyl group to the 1’-Nitrogen, yielding a caffeine molecule (Denoeud ''et al'' 2014). |
[[Image:Caffeine_mechanism.png]] | [[Image:Caffeine_mechanism.png]] | ||
| Line 22: | Line 22: | ||
== Adenosine == | == Adenosine == | ||
| - | Adenosine is an inhibitory neurotransmitter, which promotes sleep and inhibits arousal. It has two components; an adenine nucleotide and a ribose sugar. Adenosine is a polar molecule and is water soluble. Within the brain, concentration of this neuromodulator increases every hour. Adenosine binds intracellularly to G-protein and induces multiple effects. The G-protein is composed of 7 alpha helices, which provide its secondary structure, and is a transmembrane protein. As adenosine receptors bind G-protein, neural activity begins to decrease and the person feels fatigued and sleepy. A2A receptor is one of many adenosine G protein-coupled receptors. | + | Adenosine is an inhibitory neurotransmitter, which promotes sleep and inhibits arousal. It has two components; an adenine nucleotide and a ribose sugar. Adenosine is a polar molecule and is water soluble. Within the brain, concentration of this neuromodulator increases every hour. Adenosine binds intracellularly to G-protein and induces multiple effects. The G-protein is composed of 7 alpha helices, which provide its secondary structure, and is a transmembrane protein. As adenosine receptors bind G-protein, neural activity begins to decrease and the person feels fatigued and sleepy. A2A receptor is one of many adenosine G protein-coupled receptors (Huang ''et al'' 2014). |
<scene name='71/716555/Antagonist_binding/1'>A2A Receptor with Adenosine Bound</scene> | <scene name='71/716555/Antagonist_binding/1'>A2A Receptor with Adenosine Bound</scene> | ||
| Line 43: | Line 43: | ||
== Conclusion == | == Conclusion == | ||
| - | Caffeine increases temporary alertness, energy, and mood. Sensitivity to caffeine is different from person to person. It will be more effective to a small individual than a larger individual. While considered safe in small quantities, caffeine can cause irritability and headaches if someone consumes over 300 mg per day. This intake can be reduced by consumption of non-caffeinated coffee, water and tea (Dore ''et all'' 2011). Caffeine is still largely misunderstood, and in the next few years, more studies will be done on it because it is becoming a larger factor in today’s school and work place(Olsen, 2013). | + | Caffeine increases temporary alertness, energy, and mood. Sensitivity to caffeine is different from person to person. It will be more effective to a small individual than a larger individual. While considered safe in small quantities, caffeine can cause irritability and headaches if someone consumes over 300 mg per day. This intake can be reduced by consumption of non-caffeinated coffee, water and tea (Dore ''et all'' 2011). Caffeine is still largely misunderstood, and in the next few years, more studies will be done on it because it is becoming a larger factor in today’s school and work place (Olsen, 2013). |
== See Also == | == See Also == | ||
| Line 73: | Line 73: | ||
Doré, A. S. et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19, 1283–1293 (2011) | Doré, A. S. et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19, 1283–1293 (2011) | ||
| + | |||
| + | Huang, Z.L. | ||
Mitchell, Elizabeth. Caffeine: Convergently Evolved or Creatively Provided. Digital image. Answersingenesis. N.p., 20 Sept. 2014. Web. <https://answersingenesis.org/evidence-for-creation/design-in-nature/caffeine-convergently-evolved-creatively-provided/>. | Mitchell, Elizabeth. Caffeine: Convergently Evolved or Creatively Provided. Digital image. Answersingenesis. N.p., 20 Sept. 2014. Web. <https://answersingenesis.org/evidence-for-creation/design-in-nature/caffeine-convergently-evolved-creatively-provided/>. | ||
Revision as of 18:09, 20 November 2015
The Affect of Caffeine (Trimethylxanthine) on Human A2A Receptor
| |||||||||||
References
Antonioli, Luca, Corrado Blandizzi, Pal Pacher, and Gyorgy Haskó. "Adensoine and Adenosine Receptors." Nature.com. Nature Publishing Group, 2013. Web. 16 Nov. 2015.
"ADORA2B Adenosine A2b Receptor [ Homo Sapiens (human) ]." NCIB. N.p., n.d. Web. 16 Nov. 2015.
"ADORA3 Adenosine A3 Receptor [ Homo Sapiens (human) ]." NCBI. N.p., n.d. Web. 16 Nov. 2015.
Denoeud, F., L. Carretero-Paulet, A. Dereeper, G. Droc, R. Guyot, M. Pietrella, C. Zheng, A. Alberti, F. Anthony, G. Aprea, J.-M. Aury, P. Bento, M. Bernard, S. Bocs, C. Campa, A. Cenci, M.-C. Combes, D. Crouzillat, C. Da Silva, L. Daddiego, F. De Bellis, S. Dussert, O. Garsmeur, T. Gayraud, V. Guignon, K. Jahn, V. Jamilloux, T. Joet, K. Labadie, T. Lan, J. Leclercq, M. Lepelley, T. Leroy, L.-T. Li, P. Librado, L. Lopez, A. Munoz, B. Noel, A. Pallavicini, G. Perrotta, V. Poncet, D. Pot, Priyono, M. Rigoreau, M. Rouard, J. Rozas, C. Tranchant-Dubreuil, R. Vanburen, Q. Zhang, A. C. Andrade, X. Argout, B. Bertrand, A. De Kochko, G. Graziosi, R. J. Henry, Jayarama, R. Ming, C. Nagai, S. Rounsley, D. Sankoff, G. Giuliano, V. A. Albert, P. Wincker, and P. Lashermes. "The Coffee Genome Provides Insight into the Convergent Evolution of Caffeine Biosynthesis." Science 345.6201 (2014): 1181-184.
Doré, A. S. et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19, 1283–1293 (2011)
Huang, Z.L.
Mitchell, Elizabeth. Caffeine: Convergently Evolved or Creatively Provided. Digital image. Answersingenesis. N.p., 20 Sept. 2014. Web. <https://answersingenesis.org/evidence-for-creation/design-in-nature/caffeine-convergently-evolved-creatively-provided/>.
Oslen, N.L. "Caffeine Consumption Habits and Perceptions among University of New Hampshire Students" University of New Hampshire Scholars' Repository 103.1 (2013): Print.
Xanthine. Digital image. LookForDiagnosis. N.p., Sept. 2014. Web. <http://www.lookfordiagnosis.com/mesh_info.php?term=Xanthine&lang=1>.
Xu, Fei, Huizian Wu, Vsevolod Katritch, Gye Won Han, Kenneth A. Jacobson, Zhan-Guo Gao, Vadim Cherezov, and Raymond C. Stevens. "Structure of an Agonist-Bound Human A2A Adenosine Receptor." (n.d.): n. pag. Web. 8 Nov. 2015.
Xu, Fei, and Raymond C. Stevens. “Trapping Small Caffeine in a Large GPCR Pocket.” Structure (London, England : 1993) 19.9 (2011): 1204–1207. PMC. Web. 17 Nov. 2015.
Proteopedia Page Contributors and Editors (what is this?)
Mackenzie Kincer, Sidiqullah Halim, Alexander Berchansky, Michal Harel