Caffeine
From Proteopedia
| Line 11: | Line 11: | ||
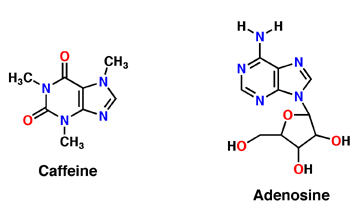
Caffeine is a derivative of adenosine and is also called Trimethylxanthine. It is composed of purines; structurally it is polar, and water soluble. They antagonize or inhibit many of the adenosine receptors, like the A1 receptor mentioned above. Caffeine affects neurons and glial cells in the brain by binding to the same location that adenosine would bind and then induce a cascade of enzymatic downstream effects (Denoeud ''et al'' 2014). | Caffeine is a derivative of adenosine and is also called Trimethylxanthine. It is composed of purines; structurally it is polar, and water soluble. They antagonize or inhibit many of the adenosine receptors, like the A1 receptor mentioned above. Caffeine affects neurons and glial cells in the brain by binding to the same location that adenosine would bind and then induce a cascade of enzymatic downstream effects (Denoeud ''et al'' 2014). | ||
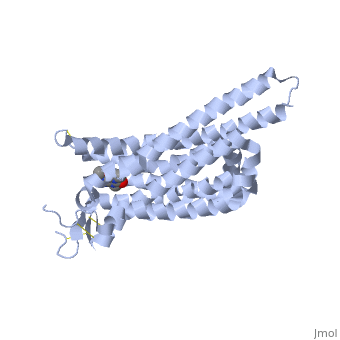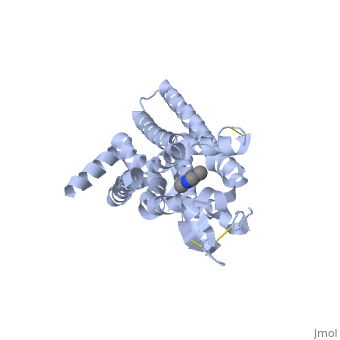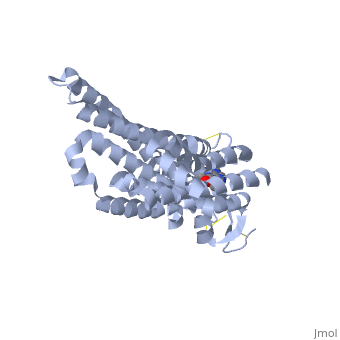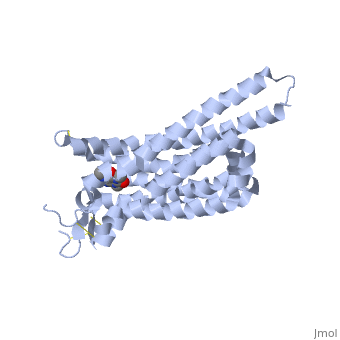
| - | <scene name='71/716555/Caffeine_binding/4'>A2A Receptor with Caffeine bound</scene> | ||
| Line 40: | Line 39: | ||
A2A is a transmembrane protein coupled with a G protein in humans. Trimethylxanthine has a highly water soluble and thus when present in the system, interacts with the A2A receptor. In order for Trimethylxantine to bind to the receptor, the third and seventh transmembrane helical domains need to recognize the ligand. Trimethylxanthine can then bind. Trimethylxanthine, due to its similar structure and its purine alkaloid structure, can bind in place of adenosine. This binding will change the shape, but not initiate the cascade of downstream effects that adenosine does, like opening of ion channels and slowing its activity. The concentrate of free adenosine increases extracellularly, when trimethylxanthine is bound (Xu and Stevens, 2011). | A2A is a transmembrane protein coupled with a G protein in humans. Trimethylxanthine has a highly water soluble and thus when present in the system, interacts with the A2A receptor. In order for Trimethylxantine to bind to the receptor, the third and seventh transmembrane helical domains need to recognize the ligand. Trimethylxanthine can then bind. Trimethylxanthine, due to its similar structure and its purine alkaloid structure, can bind in place of adenosine. This binding will change the shape, but not initiate the cascade of downstream effects that adenosine does, like opening of ion channels and slowing its activity. The concentrate of free adenosine increases extracellularly, when trimethylxanthine is bound (Xu and Stevens, 2011). | ||
cAMP levels increase when adenosine is bound and are not effected when caffeine is bound. ERK1 and ERK2 are kinases, modify serine and threonine, of the GMGC group that regulation of cell growth and differentiation, and if adenosine was bound, this cascade of events would occur, but when Trimethylxanthine is bound, this regulation does not occur (Xu and Stevens, 2011). | cAMP levels increase when adenosine is bound and are not effected when caffeine is bound. ERK1 and ERK2 are kinases, modify serine and threonine, of the GMGC group that regulation of cell growth and differentiation, and if adenosine was bound, this cascade of events would occur, but when Trimethylxanthine is bound, this regulation does not occur (Xu and Stevens, 2011). | ||
| + | |||
| + | <scene name='71/716555/Caffeine_binding/4'>A2A Receptor with Caffeine bound</scene> | ||
== Conclusion == | == Conclusion == | ||
Revision as of 01:18, 8 December 2015
The Effect of Caffeine (Trimethylxanthine) on Human A2A Receptor
| |||||||||||
References
Antonioli, Luca, Corrado Blandizzi, Pal Pacher, and Gyorgy Haskó. "Adensoine and Adenosine Receptors." Nature.com. Nature Publishing Group, 2013. Web. 16 Nov. 2015.
"ADORA2B Adenosine A2b Receptor [ Homo Sapiens (human) ]." NCIB. N.p., n.d. Web. 16 Nov. 2015.
"ADORA3 Adenosine A3 Receptor [ Homo Sapiens (human) ]." NCBI. N.p., n.d. Web. 16 Nov. 2015.
Denoeud, F., L. Carretero-Paulet, A. Dereeper, G. Droc, R. Guyot, M. Pietrella, C. Zheng, A. Alberti, F. Anthony, G. Aprea, J.-M. Aury, P. Bento, M. Bernard, S. Bocs, C. Campa, A. Cenci, M.-C. Combes, D. Crouzillat, C. Da Silva, L. Daddiego, F. De Bellis, S. Dussert, O. Garsmeur, T. Gayraud, V. Guignon, K. Jahn, V. Jamilloux, T. Joet, K. Labadie, T. Lan, J. Leclercq, M. Lepelley, T. Leroy, L.-T. Li, P. Librado, L. Lopez, A. Munoz, B. Noel, A. Pallavicini, G. Perrotta, V. Poncet, D. Pot, Priyono, M. Rigoreau, M. Rouard, J. Rozas, C. Tranchant-Dubreuil, R. Vanburen, Q. Zhang, A. C. Andrade, X. Argout, B. Bertrand, A. De Kochko, G. Graziosi, R. J. Henry, Jayarama, R. Ming, C. Nagai, S. Rounsley, D. Sankoff, G. Giuliano, V. A. Albert, P. Wincker, and P. Lashermes. "The Coffee Genome Provides Insight into the Convergent Evolution of Caffeine Biosynthesis." Science 345.6201 (2014): 1181-184.
Doré, A. S. et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19, 1283–1293 (2011)
Huang, Z.L., Zhang, Z. and Qu, W.M. "Roles of adenosine and its receptors in sleep-wake regulation." International Review Neurobiology 119.001 (2014): 349-371.
Mitchell, Elizabeth. Caffeine: Convergently Evolved or Creatively Provided. Digital image. Answersingenesis. N.p., 20 Sept. 2014. Web. <https://answersingenesis.org/evidence-for-creation/design-in-nature/caffeine-convergently-evolved-creatively-provided/>.
Oslen, N.L. "Caffeine Consumption Habits and Perceptions among University of New Hampshire Students" University of New Hampshire Scholars' Repository 103.1 (2013): Print.
Xanthine. Digital image. LookForDiagnosis. N.p., Sept. 2014. Web. <http://www.lookfordiagnosis.com/mesh_info.php?term=Xanthine&lang=1>.
Xu, Fei, Huizian Wu, Vsevolod Katritch, Gye Won Han, Kenneth A. Jacobson, Zhan-Guo Gao, Vadim Cherezov, and Raymond C. Stevens. "Structure of an Agonist-Bound Human A2A Adenosine Receptor." (n.d.): n. pag. Web. 8 Nov. 2015.
Xu, Fei, and Raymond C. Stevens. “Trapping Small Caffeine in a Large GPCR Pocket.” Structure (London, England : 1993) 19.9 (2011): 1204–1207. PMC. Web. 17 Nov. 2015.
Proteopedia Page Contributors and Editors (what is this?)
Mackenzie Kincer, Sidiqullah Halim, Alexander Berchansky, Michal Harel