This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1123
From Proteopedia
| Line 1: | Line 1: | ||
| - | <Structure load='3H47' size=' | + | <Structure load='3H47' size='370' frame='false' align='right' caption='Structure of Human Immunodeficiency Virus monomeric capsid PDB:3H47' scene='71/719864/Hiv_capsid_monomer/1' /> |
| - | <Structure load='3J4F' size=' | + | <Structure load='3J4F' size='370' frame='false' align='right' caption='Structure of Human Immunodeficiency Virus hexameric capsid PDB:3H47' scene='71/719864/Hiv_capsid_hexamer/1' /> |
<scene name='71/719864/Hiv_capsid_hexamer/1'>HIV capsid hexamer </scene> | <scene name='71/719864/Hiv_capsid_hexamer/1'>HIV capsid hexamer </scene> | ||
Revision as of 16:11, 28 January 2016
|
|
Contents |
Introduction
Function
Structural highlights
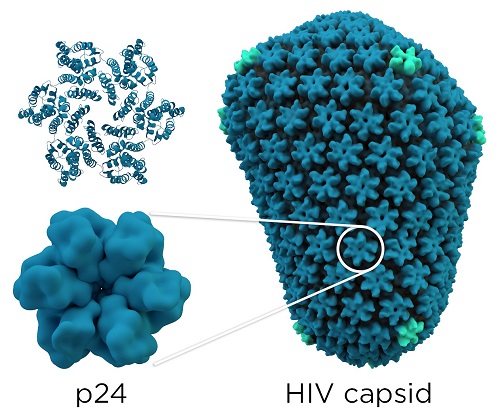
As you can see on the figure bellow, each monomer of capsid is linked to five others to form a hexamer. These hexamers (approximately 330 per virus) associates themselves to form a non-symetrical protein complex.
Interactions with others partners
Even if p24 is classified as a structural protein, it is also involved in many cellular infection processes.
You can find bellow in a non exhaustive list of p24 partners :
- Cyclophylin A [3]
The HIV-1 capsid acts like a kind of "nuclear localisation signal" because it targets directly the virus toward the nucleus, where the integration takes place.
Capsid as therapeutical target
|
Thank to its central role in viral infectious process (genome protection, enveloppe cohesion, uncoating, budding), HIV capsid is a very good target for antiviral drugs. As a result, many research teams are working in this way and some molecules such as [4] are very promising. By binding to a "central pocket" on P24, it was shown in vitro that this compound is inhibiting the new viruses assembly, and consequently the virus budding.
This approach is interesting because, based on structural information, we are able to build such ligands using drug design. However, we are still far away from the "miraculous HIV drug", because the pathway from design to approved drug is not an easy way at all.
References
- ↑ Fernandez J, Gärtner K, Becker A, et al. HIV-1 capsid interacts with cytoskeletal-associated proteins for intracytoplasmic routing to the nucleus. Retrovirology. 2013;10(Suppl 1):P34. doi:10.1186/1742-4690-10-S1-P34.
- ↑ Fernandez J, Portilho DM, Danckaert A, Munier S, Becker A, Roux P, Zambo A, Shorte S, Jacob Y, Vidalain PO, Charneau P, Clavel F, Arhel NJ. Microtubule-associated proteins 1 (MAP1) promote human immunodeficiency virus type I (HIV-1) intracytoplasmic routing to the nucleus. J Biol Chem. 2015 Feb 20;290(8):4631-46. doi: 10.1074/jbc.M114.613133. Epub 2014 Dec 11.
- ↑ [http://jvi.asm.org/content/84/10/5181.long Marisa S. Briones, Charles W. Dobard and Samson A. Chow. Role of Human Immunodeficiency Virus Type 1 Integrase in Uncoating of the Viral Core. Accepted manuscript posted online 10 March 2010, doi:.1128/JVI.02382-09 J. Virol. May 2010 vol. 84 no. 10 5181-5190]
- ↑ 4.0 4.1 4.2 HIV-1 capsid: the multifaceted key player in HIV-1 infection Nature Reviews Microbiology 13,471–483 (2015)doi:10.1038/nrmicro3503
Structural capsid image : By Thomas Splettstoesser (www.scistyle.com) (Own work) [CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons