We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Journal:Molecular Cell:1
From Proteopedia
(Difference between revisions)

| Line 25: | Line 25: | ||
Comparison of the <span style="color:yellow;background-color:black;font-weight:bold;">dAChE4 design model (yellow)</span> with the <span style="color:lime;background-color:black;font-weight:bold;">solved crystal structure (PDB entry: [[5hq3]], green)</span> and <span style="color:violet;background-color:black;font-weight:bold;">(wild-type hAChE (PDB entry: [[4ey4]], violet)</span>: | Comparison of the <span style="color:yellow;background-color:black;font-weight:bold;">dAChE4 design model (yellow)</span> with the <span style="color:lime;background-color:black;font-weight:bold;">solved crystal structure (PDB entry: [[5hq3]], green)</span> and <span style="color:violet;background-color:black;font-weight:bold;">(wild-type hAChE (PDB entry: [[4ey4]], violet)</span>: | ||
| - | *<scene name='72/728277/Cv/14'>Overall | + | *<scene name='72/728277/Cv/14'>Overall alignment</scene>. |
*<scene name='72/728277/Cv/13'>Sub-Ångstrom accuracy in design of 2 small-to-large core mutations</scene>. | *<scene name='72/728277/Cv/13'>Sub-Ångstrom accuracy in design of 2 small-to-large core mutations</scene>. | ||
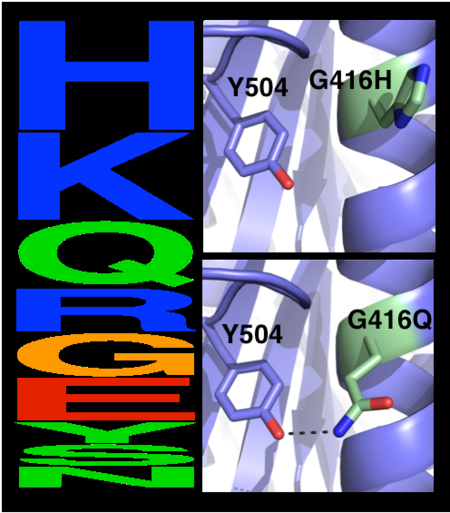
*<scene name='72/728277/Cv/19'>The maximal deviation observed between any respective Cα atoms</scene> in the model and structure is 3.1 Å (dashed line). This conformation change likely results from elimination of a side chain-backbone hydrogen bond between Thr112 and Ser110 due to the designed Thr112Ala mutation. | *<scene name='72/728277/Cv/19'>The maximal deviation observed between any respective Cα atoms</scene> in the model and structure is 3.1 Å (dashed line). This conformation change likely results from elimination of a side chain-backbone hydrogen bond between Thr112 and Ser110 due to the designed Thr112Ala mutation. | ||
Revision as of 16:20, 3 May 2016
| |||||||||||
- ↑ REF
Proteopedia Page Contributors and Editors (what is this?)
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.