CRISPR-Cas9
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
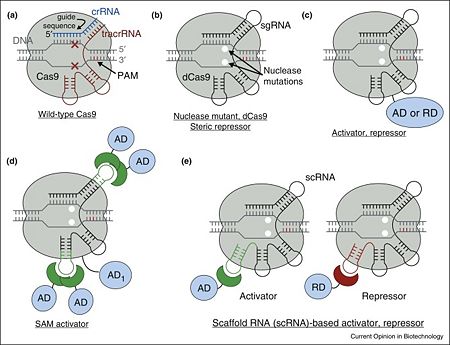
Cas9 is a key protein of bacterial Type II CRISPR adaptive immune system. In its native context, Cas9 is an RNA-guided endonuclease that is responsible for targeted degradation of the invading foreign DNA–plasmids and phages. Cas9 is directed to its DNA targets by forming a ribonucleoprotein complex with two small non-coding RNAs: CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA) (Figure 1a). By elegant engineering, crRNA and tracrRNA can be joined end-to-end and transcribed as a single guide RNA (sgRNA) that too efficiently directs Cas9 protein to DNA targets encoded within the guide sequence of sgRNA <ref name="Jinek">PMID:22745249</ref>. The optimal DNA target of the complex is determined by a Watson–Crick base pairing of a short ∼20-nt sequence within sgRNA (within the crRNA in wild-type), termed the guide sequence, adjacent to a few nucleotide long conserved motif recognized directly by Cas9 protein (protospacer adjacent motif, PAM) <ref name="Jinek">PMID:22745249</ref>. Despite this, a few mismatches between guide sequence and target DNA can be tolerated <ref name="Jinek">PMID:22745249</ref>, more so within the 5’ proximal position of the guide sequence. Cas9 nuclease can be converted into deactivated Cas9 (dCas9), an RNA-programmable DNA-binding protein, by mutating two key residues within its nuclease domains (Figure 1b) <ref name="Did">PMID:27344519</ref><ref name="Jinek">PMID:22745249</ref>. | Cas9 is a key protein of bacterial Type II CRISPR adaptive immune system. In its native context, Cas9 is an RNA-guided endonuclease that is responsible for targeted degradation of the invading foreign DNA–plasmids and phages. Cas9 is directed to its DNA targets by forming a ribonucleoprotein complex with two small non-coding RNAs: CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA) (Figure 1a). By elegant engineering, crRNA and tracrRNA can be joined end-to-end and transcribed as a single guide RNA (sgRNA) that too efficiently directs Cas9 protein to DNA targets encoded within the guide sequence of sgRNA <ref name="Jinek">PMID:22745249</ref>. The optimal DNA target of the complex is determined by a Watson–Crick base pairing of a short ∼20-nt sequence within sgRNA (within the crRNA in wild-type), termed the guide sequence, adjacent to a few nucleotide long conserved motif recognized directly by Cas9 protein (protospacer adjacent motif, PAM) <ref name="Jinek">PMID:22745249</ref>. Despite this, a few mismatches between guide sequence and target DNA can be tolerated <ref name="Jinek">PMID:22745249</ref>, more so within the 5’ proximal position of the guide sequence. Cas9 nuclease can be converted into deactivated Cas9 (dCas9), an RNA-programmable DNA-binding protein, by mutating two key residues within its nuclease domains (Figure 1b) <ref name="Did">PMID:27344519</ref><ref name="Jinek">PMID:22745249</ref>. | ||
| - | ====Examples of 3D structures of crRNA==== | + | ====Examples of 3D structures of CRISPR RNA (crRNA)==== |
*<scene name='74/742625/Cv3/1'>CRISPR-Cas Cpf1 endonuclease-crRNA-DNA ternary complex</scene> from ''Acidaminococcus sp. BV3L6'' ([[5kk5]]). | *<scene name='74/742625/Cv3/1'>CRISPR-Cas Cpf1 endonuclease-crRNA-DNA ternary complex</scene> from ''Acidaminococcus sp. BV3L6'' ([[5kk5]]). | ||
*<scene name='74/742625/Cv2/10'>Crystal structure of Acidaminococcus sp. Cpf1 in complex with crRNA and target DNA</scene> ([[5b43]]). | *<scene name='74/742625/Cv2/10'>Crystal structure of Acidaminococcus sp. Cpf1 in complex with crRNA and target DNA</scene> ([[5b43]]). | ||
*<scene name='74/742625/Cv2/9'>crRNA-dsDNA hybrid from E. coli</scene> ([[5h9f]]). | *<scene name='74/742625/Cv2/9'>crRNA-dsDNA hybrid from E. coli</scene> ([[5h9f]]). | ||
| - | + | *<scene name='74/742625/Cv2/7'>crRNA-dsDNA hybrid and Cascade proteins from E. coli</scene> ([[4u7u]]). | |
| + | |||
| + | ====Examples of 3D structures of single guide RNA (sgRNA)==== | ||
| + | |||
==Crystal structure of a CRISPR RNA-guided surveillance complex, Cascade, bound to a ssDNA target<ref>PMID:25123481</ref>== | ==Crystal structure of a CRISPR RNA-guided surveillance complex, Cascade, bound to a ssDNA target<ref>PMID:25123481</ref>== | ||
The <scene name='74/742625/Cv/5'>crystal structure of ssDNA-bound Cascade has the seahorse architecture</scene>. The body is formed by a helical filament of six Cas7 subunits (Cas7.1 to 7.6) wrapped around the crRNA guide, with a head-to-tail dimer of Cse2 (Cse2.1 and Cse2.2) at the belly. Cas6e and the 3′ handle of crRNA cap the Cas7 filament at the head while Cas5 and the 5′ handle cap the tail. The N-terminal base of Cse1 is positioned at the tail of the filament; the C-terminal four-helix bundle contacts Cse2.2. The ssDNA target is juxtaposed to the guide region of the crRNA in a groove formed by the Cas7 filament, the four-helix bundle of Cse1, and the Cse2 dimer. | The <scene name='74/742625/Cv/5'>crystal structure of ssDNA-bound Cascade has the seahorse architecture</scene>. The body is formed by a helical filament of six Cas7 subunits (Cas7.1 to 7.6) wrapped around the crRNA guide, with a head-to-tail dimer of Cse2 (Cse2.1 and Cse2.2) at the belly. Cas6e and the 3′ handle of crRNA cap the Cas7 filament at the head while Cas5 and the 5′ handle cap the tail. The N-terminal base of Cse1 is positioned at the tail of the filament; the C-terminal four-helix bundle contacts Cse2.2. The ssDNA target is juxtaposed to the guide region of the crRNA in a groove formed by the Cas7 filament, the four-helix bundle of Cse1, and the Cse2 dimer. | ||
Revision as of 08:32, 6 October 2016
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Didovyk A, Borek B, Tsimring L, Hasty J. Transcriptional regulation with CRISPR-Cas9: principles, advances, and applications. Curr Opin Biotechnol. 2016 Aug;40:177-84. doi: 10.1016/j.copbio.2016.06.003. Epub, 2016 Jun 23. PMID:27344519 doi:http://dx.doi.org/10.1016/j.copbio.2016.06.003
- ↑ 2.0 2.1 2.2 2.3 Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012 Aug 17;337(6096):816-21. doi: 10.1126/science.1225829. Epub 2012, Jun 28. PMID:22745249 doi:http://dx.doi.org/10.1126/science.1225829
- ↑ Mulepati S, Heroux A, Bailey S. Crystal structure of a CRISPR RNA-guided surveillance complex bound to a ssDNA target. Science. 2014 Aug 14. pii: 1256996. PMID:25123481 doi:http://dx.doi.org/10.1126/science.1256996
- ↑ Hayes RP, Xiao Y, Ding F, van Erp PB, Rajashankar K, Bailey S, Wiedenheft B, Ke A. Structural basis for promiscuous PAM recognition in type I-E Cascade from E. coli. Nature. 2016 Feb 25;530(7591):499-503. doi: 10.1038/nature16995. Epub 2016 Feb, 10. PMID:26863189 doi:http://dx.doi.org/10.1038/nature16995