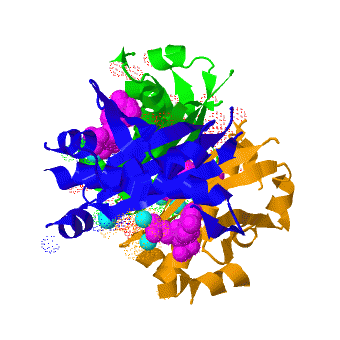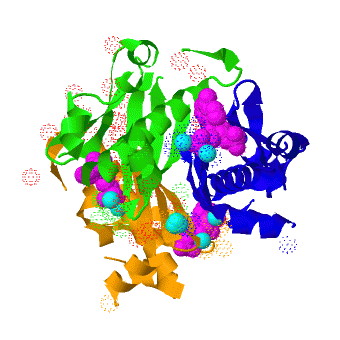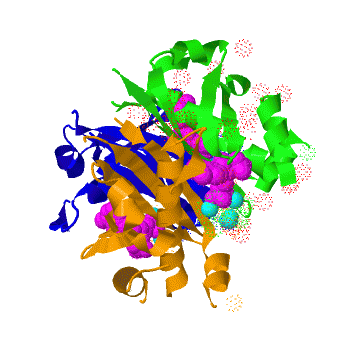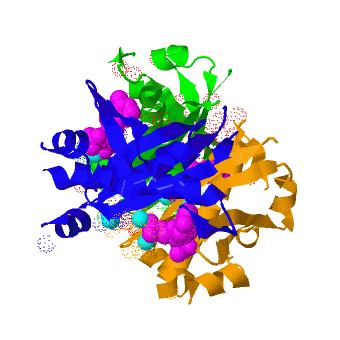
Acyl carrier protein synthase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='350' scene='3hqj/Trimer/1' caption="Acyl carrier protein synthase complex with CoA and Mg+2 ion (cyan) [[3hqj]]" > |
The crystal structure of '''Acyl carrier protein synthase (AcpS)''' from [http://http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''Mycobacterium tuberculosis''] (''Mtb'') was solved at 1.95 Å ([[3hqj]]). It crystallized as one <scene name='3hqj/Trimer/2'>monomer</scene> per asymmetric unit. Since ''Mtb'' AcpS has biologically active trimeric arrangement, <scene name='3hqj/Trimer/3'>AcpS trimer</scene> (in <span style="color:lime;background-color:black;font-weight:bold;">green</span>, <font color='blue'><b>blue</b></font>, and <scene name='3hqj/Trimer/3'>AcpS trimer</scene> (in <span style="color:orange;background-color:black;font-weight:bold;">orange</span>) was constructed using the 3-fold crystallographic symmetry in the ''P''23 space group. | The crystal structure of '''Acyl carrier protein synthase (AcpS)''' from [http://http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''Mycobacterium tuberculosis''] (''Mtb'') was solved at 1.95 Å ([[3hqj]]). It crystallized as one <scene name='3hqj/Trimer/2'>monomer</scene> per asymmetric unit. Since ''Mtb'' AcpS has biologically active trimeric arrangement, <scene name='3hqj/Trimer/3'>AcpS trimer</scene> (in <span style="color:lime;background-color:black;font-weight:bold;">green</span>, <font color='blue'><b>blue</b></font>, and <scene name='3hqj/Trimer/3'>AcpS trimer</scene> (in <span style="color:orange;background-color:black;font-weight:bold;">orange</span>) was constructed using the 3-fold crystallographic symmetry in the ''P''23 space group. | ||
Revision as of 14:22, 3 October 2017
| |||||||||||
3D structures of acyl carrier protein synthase
Updated on 03-October-2017
References
- Parris KD, Lin L, Tam A, Mathew R, Hixon J, Stahl M, Fritz CC, Seehra J, Somers WS. Crystal structures of substrate binding to Bacillus subtilis holo-(acyl carrier protein) synthase reveal a novel trimeric arrangement of molecules resulting in three active sites. Structure. 2000 Aug 15;8(8):883-95. PMID:10997907
- Dym O, Albeck S, Peleg Y, Schwarz A, Shakked Z, Burstein Y, Zimhony O. Structure-function analysis of the acyl carrier protein synthase (AcpS) from Mycobacterium tuberculosis. J Mol Biol. 2009 Nov 6;393(4):937-50. Epub 2009 Sep 3. PMID:19733180 doi:10.1016/j.jmb.2009.08.065
Proteopedia Page Contributors and Editors (what is this?)
Categories: Topic Page | Mycobacterium tuberculosis | Albeck,S. | Burstein,Y. | Dym,O. | ISPC, Israel Structural Proteomics Center. | Peleg,Y. | Schwarz,A. | Shakked,Z. | Zimhony,O. | An/fold | Cytoplasm | Fatty acid biosynthesis | ISPC | Israel Structural Proteomics Center | Lipid synthesis | Magnesium | Metal-binding | Structural genomic | Transferase