We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox k11v
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
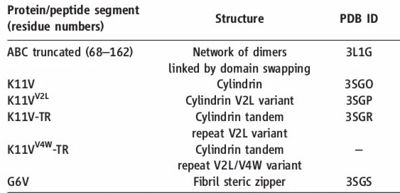
The entire 11-residue segment KVKVLGDVIEV forms a <scene name='77/771966/K11v_in_black/1'>hairpin loop</scene> in the 3D structure of ABC.The six residue segment GDVIEV ,termed <scene name='77/771966/Gdviev_black/1'>G6V</scene>,forms fibrils and microcrystals.The microcrystals enabled us to determine the atomic structure of G6V, which proved to be a standard class 2 steric zipper, essentially an amyloid-like protofilament. | The entire 11-residue segment KVKVLGDVIEV forms a <scene name='77/771966/K11v_in_black/1'>hairpin loop</scene> in the 3D structure of ABC.The six residue segment GDVIEV ,termed <scene name='77/771966/Gdviev_black/1'>G6V</scene>,forms fibrils and microcrystals.The microcrystals enabled us to determine the atomic structure of G6V, which proved to be a standard class 2 steric zipper, essentially an amyloid-like protofilament. | ||
| - | Amyloid fibrils and oligomer are both formed by the hairpin segment KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to K11V^V2L(K11V-TR).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC. | + | Amyloid fibrils and oligomer are both formed by the hairpin segment KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to K11V^V2L(K11V-TR).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2(K11V^2L) forms stable oligomers intermediate in size between monomer and fiber. |
| + | |||
| + | K11V and K11V^V2L form hexameric oligomers.K11V oligomer is of 6 chains and K11V-TR oligomer of three tandem chains. | ||
| + | |||
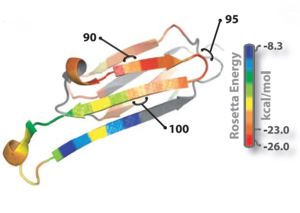
| + | The structure of K11V is a six-stranded antiparallel barrel of cylindrical in shape also called as cylindrin.Each strand of cylindrin is bonded to one neighbouring strand by a strong interface and to a second by a weak interface.The weak interface is formed by eight hydrogen bonds: four from the main chain, two mediated through side-chain interactions, and two through a water bridge.The strong interface is formed by 12 hydrogen bonds and spreads outward at the ends. | ||
[[Image:Table_abc_first_ok.jpg | thumb | 400px | centre | Information about amyloid related oligomers,derived from ABC ]] | [[Image:Table_abc_first_ok.jpg | thumb | 400px | centre | Information about amyloid related oligomers,derived from ABC ]] | ||
Revision as of 14:32, 9 November 2017
Toxic Amyloid Small Oligomer’s atomic view
| |||||||||||
References
- ↑ Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, Cascio D, Glabe C, Eisenberg D. Atomic view of a toxic amyloid small oligomer. Science. 2012 Mar 9;335(6073):1228-31. PMID:22403391 doi:10.1126/science.1213151