We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox k11v
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
Amyloid fibrils and oligomer are both formed by the hairpin segment KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to K11V^V2L(K11V-TR).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2(K11V^2L) forms stable oligomers intermediate in size between monomer and fiber. | Amyloid fibrils and oligomer are both formed by the hairpin segment KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to K11V^V2L(K11V-TR).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2(K11V^2L) forms stable oligomers intermediate in size between monomer and fiber. | ||
| - | K11V and K11V^V2L form hexameric oligomers.K11V oligomer is of 6 chains and K11V-TR oligomer of three tandem chains. | + | K11V and K11V^V2L form hexameric oligomers.K11V oligomer is of 6 chains and K11V-TR oligomer of three tandem chains.Other than the glycine linkers and the Val-to-Leu replacement, the cylindrical bodies of the six stranded K11V and the three double stranded K11V-TR oligomers are essentially identical. |
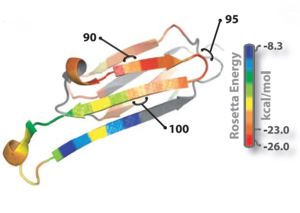
The structure of K11V is a six-stranded antiparallel barrel of cylindrical in shape also called as cylindrin.Each strand of cylindrin is bonded to one neighbouring strand by a strong interface and to a second by a weak interface.The weak interface is formed by eight hydrogen bonds: four from the main chain, two mediated through side-chain interactions, and two through a water bridge.The strong interface is formed by 12 hydrogen bonds and spreads outward at the ends. | The structure of K11V is a six-stranded antiparallel barrel of cylindrical in shape also called as cylindrin.Each strand of cylindrin is bonded to one neighbouring strand by a strong interface and to a second by a weak interface.The weak interface is formed by eight hydrogen bonds: four from the main chain, two mediated through side-chain interactions, and two through a water bridge.The strong interface is formed by 12 hydrogen bonds and spreads outward at the ends. | ||
| + | |||
| + | There are proofs that amyloid oligomers are beta-sheet rich,and many toxic oligomers are recognized by A11 conformational antibody,which also recognizes cylindrin.Threfore, the cylindrin structure may represent amyloid oligomer's common structural core. | ||
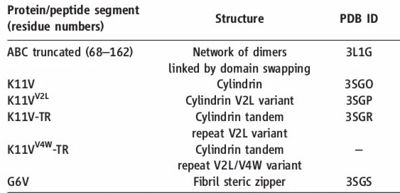
[[Image:Table_abc_first_ok.jpg | thumb | 400px | centre | Information about amyloid related oligomers,derived from ABC ]] | [[Image:Table_abc_first_ok.jpg | thumb | 400px | centre | Information about amyloid related oligomers,derived from ABC ]] | ||
Revision as of 14:46, 9 November 2017
Toxic Amyloid Small Oligomer’s atomic view
| |||||||||||
References
- ↑ Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, Cascio D, Glabe C, Eisenberg D. Atomic view of a toxic amyloid small oligomer. Science. 2012 Mar 9;335(6073):1228-31. PMID:22403391 doi:10.1126/science.1213151