We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox k11v
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
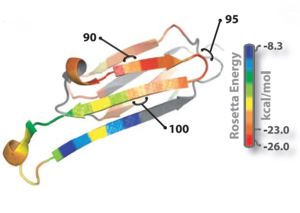
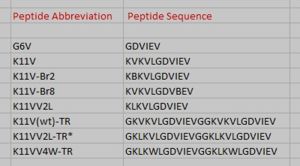
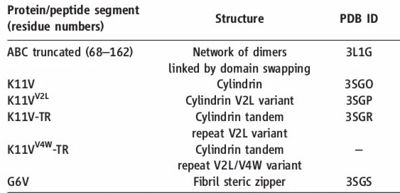
The entire 11-residue segment KVKVLGDVIEV forms a <scene name='77/771966/K11v_in_black/2'>hairpin loop</scene> in the 3D structure of ABC.The six residue segment GDVIEV ,termed <scene name='77/771966/Gdviev_black/1'>G6V</scene>,forms fibrils and microcrystals.The microcrystals enabled us to determine the atomic structure of G6V, which proved to be a standard class 2 steric zipper, essentially an amyloid-like protofilament. | The entire 11-residue segment KVKVLGDVIEV forms a <scene name='77/771966/K11v_in_black/2'>hairpin loop</scene> in the 3D structure of ABC.The six residue segment GDVIEV ,termed <scene name='77/771966/Gdviev_black/1'>G6V</scene>,forms fibrils and microcrystals.The microcrystals enabled us to determine the atomic structure of G6V, which proved to be a standard class 2 steric zipper, essentially an amyloid-like protofilament. | ||
| - | Amyloid fibrils and oligomer are both formed by the <scene name='77/771966/K11v_in_black/2'>hairpin segment</scene> KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to <scene name='77/771966/K11v-v2l3sgp/1'>K11V<sup>V2L</sup></scene>(<scene name='77/771966/K11v-tr3sgr/1'>K11V-TR</scene>).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2(K11V<sup>2L</sup>) are capable of converting to amyloid state as their parent protein ABC and forms stable oligomers intermediate in size between monomer and fiber. | + | Amyloid fibrils and oligomer are both formed by the <scene name='77/771966/K11v_in_black/2'>hairpin segment</scene> KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to <scene name='77/771966/K11v-v2l3sgp/1'>K11V<sup>V2L</sup></scene>(<scene name='77/771966/K11v-tr3sgr/1'>K11V-TR</scene>).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2( K11V<sup>2L</sup> ) are capable of converting to amyloid state as their parent protein ABC and forms stable oligomers intermediate in size between monomer and fiber. |
ABC K11V oligomers exhibit molecular properties in common with amyloid oligomers from other disease-related proteins and oligomers were were observed to be toxic ,displaying dose-response effects similar to those of alpha-beta involved in Alzheimer's disease. | ABC K11V oligomers exhibit molecular properties in common with amyloid oligomers from other disease-related proteins and oligomers were were observed to be toxic ,displaying dose-response effects similar to those of alpha-beta involved in Alzheimer's disease. | ||
Revision as of 04:34, 15 November 2017
Toxic Amyloid Small Oligomer’s atomic view
| |||||||||||
References
- ↑ Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, Cascio D, Glabe C, Eisenberg D. Atomic view of a toxic amyloid small oligomer. Science. 2012 Mar 9;335(6073):1228-31. PMID:22403391 doi:10.1126/science.1213151