We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox k11v
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
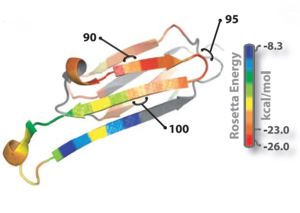
[[Image:Rosetta_image.jpg | thumb | 300px | centre | Fig. 1 : Ribbon diagram of a single subunit of ABC (16), colored by propensity to form amyloid, with red being the highest and blue the lowest propensity. The segment from residue 90 to 100, termed K11V, forms the cylindrin. ]] | [[Image:Rosetta_image.jpg | thumb | 300px | centre | Fig. 1 : Ribbon diagram of a single subunit of ABC (16), colored by propensity to form amyloid, with red being the highest and blue the lowest propensity. The segment from residue 90 to 100, termed K11V, forms the cylindrin. ]] | ||
| - | [[Image:Names_all.jpg | thumb | 300px | left | Fig. 2 : Cylindrin single chain and tandem repeat peptide abbreviations and amino acid | ||
| - | sequences. ]] | ||
The entire 11-residue segment KVKVLGDVIEV forms a <scene name='77/771966/K11v_in_black/2'>hairpin loop</scene> in the 3D structure of ABC.The six residue segment GDVIEV ,termed <scene name='77/771966/Gdviev_black/1'>G6V</scene>,forms fibrils and microcrystals.The microcrystals enabled us to determine the atomic structure of G6V, which proved to be a standard class 2 steric zipper, essentially an amyloid-like protofilament. | The entire 11-residue segment KVKVLGDVIEV forms a <scene name='77/771966/K11v_in_black/2'>hairpin loop</scene> in the 3D structure of ABC.The six residue segment GDVIEV ,termed <scene name='77/771966/Gdviev_black/1'>G6V</scene>,forms fibrils and microcrystals.The microcrystals enabled us to determine the atomic structure of G6V, which proved to be a standard class 2 steric zipper, essentially an amyloid-like protofilament. | ||
| Line 19: | Line 17: | ||
Amyloid fibrils and oligomer are both formed by the <scene name='77/771966/K11v_in_black/2'>hairpin segment</scene> KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to K11V<sup>V2L</sup> (<scene name='77/771966/K11v-tr3sgr/1'>K11V-TR</scene>).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2( K11V<sup>V2L</sup> ) are capable of converting to amyloid state as their parent protein ABC and forms stable oligomers intermediate in size between monomer and fiber. | Amyloid fibrils and oligomer are both formed by the <scene name='77/771966/K11v_in_black/2'>hairpin segment</scene> KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to K11V<sup>V2L</sup> (<scene name='77/771966/K11v-tr3sgr/1'>K11V-TR</scene>).The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2( K11V<sup>V2L</sup> ) are capable of converting to amyloid state as their parent protein ABC and forms stable oligomers intermediate in size between monomer and fiber. | ||
| - | ABC K11V oligomers exhibit molecular properties in common with amyloid oligomers from other disease-related proteins and oligomers | + | ABC K11V oligomers exhibit molecular properties in common with amyloid oligomers from other disease-related proteins and oligomers were observed to be toxic ,displaying dose-response effects similar to those of alpha-beta involved in Alzheimer's disease. |
K11V and K11V<sup>V2L</sup> form hexameric oligomers.K11V oligomer is of 6 chains and K11V-TR oligomer of three tandem chains.Other than the glycine linkers and the Val-to-Leu replacement, the cylindrical bodies of the six stranded K11V and the three double stranded K11V-TR oligomers are essentially identical. | K11V and K11V<sup>V2L</sup> form hexameric oligomers.K11V oligomer is of 6 chains and K11V-TR oligomer of three tandem chains.Other than the glycine linkers and the Val-to-Leu replacement, the cylindrical bodies of the six stranded K11V and the three double stranded K11V-TR oligomers are essentially identical. | ||
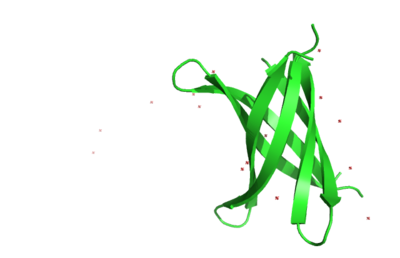
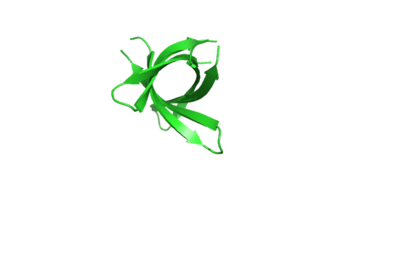
The structure of K11V is a six-stranded antiparallel barrel of cylindrical in shape also called as cylindrin(Fig. 3).It is completely different in structure from either the native structure of ABC or from G6V segment.Each strand of cylindrin is bonded to one neighbouring strand by a strong interface and to a second by a weak interface.The weak interface is formed by eight hydrogen bonds: four from the main chain, two mediated through side-chain interactions, and two through a water bridge.The strong interface is formed by 12 hydrogen bonds and spreads outward at the ends. | The structure of K11V is a six-stranded antiparallel barrel of cylindrical in shape also called as cylindrin(Fig. 3).It is completely different in structure from either the native structure of ABC or from G6V segment.Each strand of cylindrin is bonded to one neighbouring strand by a strong interface and to a second by a weak interface.The weak interface is formed by eight hydrogen bonds: four from the main chain, two mediated through side-chain interactions, and two through a water bridge.The strong interface is formed by 12 hydrogen bonds and spreads outward at the ends. | ||
| + | |||
| + | [[Image:Ribbonrepresentationofcrystalcylindrinfig2b.jpg | thumb | 400px | centre | Fig. 3 Ribbon representation of the cylindrin crystal structure. ]] | ||
THe structure of K11V-TR was determined, even though glycine linkers and Val-to-Leu replacement,cylindrical bodies of three double stranded K11V-TR oligomers and six-stranded K11V were identical. | THe structure of K11V-TR was determined, even though glycine linkers and Val-to-Leu replacement,cylindrical bodies of three double stranded K11V-TR oligomers and six-stranded K11V were identical. | ||
| Line 34: | Line 34: | ||
K11V (<scene name='77/771966/3sgo/1'>3SGO</scene>), K11V-Br2 (<scene name='77/771966/3sgm/1'>3SGM</scene>), K11V-Br8 (<scene name='77/771966/3sgn/1'>3SGN</scene>), K11VV2L (<scene name='77/771966/3sgp/1'>3SGP</scene>), K11V-TR (<scene name='77/771966/3sgr/1'>3SGR</scene>), and GDVIEV (<scene name='77/771966/3sgs/1'>3SGS</scene>). | K11V (<scene name='77/771966/3sgo/1'>3SGO</scene>), K11V-Br2 (<scene name='77/771966/3sgm/1'>3SGM</scene>), K11V-Br8 (<scene name='77/771966/3sgn/1'>3SGN</scene>), K11VV2L (<scene name='77/771966/3sgp/1'>3SGP</scene>), K11V-TR (<scene name='77/771966/3sgr/1'>3SGR</scene>), and GDVIEV (<scene name='77/771966/3sgs/1'>3SGS</scene>). | ||
| - | + | ||
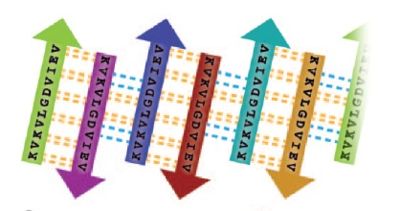
[[Image:Unrolledcylindrin.jpg | thumb | 400px | centre | Schematic of unrolled cylindrin (outside view), illustrating strand-to-strand registration. Hydrogen bonds between the main chains of neighboring strands are shown by yellow dashed lines; hydrogen bonds mediated by water bridges or side chains are shown by blue dashed lines. ]] | [[Image:Unrolledcylindrin.jpg | thumb | 400px | centre | Schematic of unrolled cylindrin (outside view), illustrating strand-to-strand registration. Hydrogen bonds between the main chains of neighboring strands are shown by yellow dashed lines; hydrogen bonds mediated by water bridges or side chains are shown by blue dashed lines. ]] | ||
[[Image:K11vtrsideview.png | thumb | 400px | centre | K11V-TR sideview ]] | [[Image:K11vtrsideview.png | thumb | 400px | centre | K11V-TR sideview ]] | ||
[[Image:Trtopview.png | thumb | 400px | centre | K11V-TR Top view ]] | [[Image:Trtopview.png | thumb | 400px | centre | K11V-TR Top view ]] | ||
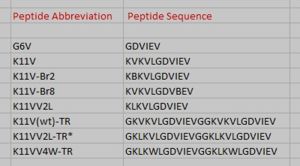
| + | [[Image:Names_all.jpg | thumb | 300px | left | Fig. 2 : Cylindrin single chain and tandem repeat peptide abbreviations and amino acid sequences. ]] | ||
| + | |||
Revision as of 07:13, 15 November 2017
Toxic Amyloid Small Oligomer’s atomic view
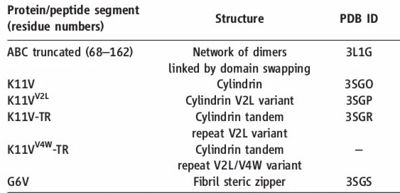
| |||||||||||
References
- ↑ Laganowsky A, Liu C, Sawaya MR, Whitelegge JP, Park J, Zhao M, Pensalfini A, Soriaga AB, Landau M, Teng PK, Cascio D, Glabe C, Eisenberg D. Atomic view of a toxic amyloid small oligomer. Science. 2012 Mar 9;335(6073):1228-31. PMID:22403391 doi:10.1126/science.1213151