Helicase
From Proteopedia
| Line 79: | Line 79: | ||
**[[2q7t]], [[2q7u]] – EcHel I relaxase domain (mutant) <BR /> | **[[2q7t]], [[2q7u]] – EcHel I relaxase domain (mutant) <BR /> | ||
**[[1p4d]] - EcHel I relaxase domain<BR /> | **[[1p4d]] - EcHel I relaxase domain<BR /> | ||
| - | **[[4n8o]] - EcHel I + DNA<br /> | + | **[[4n8o]], [[5n8o]] - EcHel I + DNA<br /> |
**[[2a0i]] - EcHel I (mutant) + DNA<BR /> | **[[2a0i]] - EcHel I (mutant) + DNA<BR /> | ||
| - | **[[1q2z]], [[1rw2]] – hHel II KU86 C terminal – NMR<BR /> | ||
**[[1e0j]], [[1e0k]] – Hel 4D domain – Phage T7 | **[[1e0j]], [[1e0k]] – Hel 4D domain – Phage T7 | ||
| Line 90: | Line 89: | ||
**[[2is4]], [[2is6]] - EcHel II (mutant) + ADP + DNA<BR /> | **[[2is4]], [[2is6]] - EcHel II (mutant) + ADP + DNA<BR /> | ||
**[[4c2t]], [[4c2u]], [[4c30]] – DrHel II + DNA - ''Deinococcus radiodurans''<br /> | **[[4c2t]], [[4c2u]], [[4c30]] – DrHel II + DNA - ''Deinococcus radiodurans''<br /> | ||
| + | **[[1q2z]], [[1rw2]] – hHel II KU86 C terminal – NMR<BR /> | ||
| + | **[[5y58]] – yHel II + DNA – yeast<br /> | ||
| + | **[[5y59]] – yHel II subunit 2 + Sir4P peptide<br /> | ||
*ATP-dependent helicase ATRX | *ATP-dependent helicase ATRX | ||
| - | **[[3qln]] - hATRX ADD domain – human<BR /> | + | **[[3qln]] - hATRX ADD domain residues 167-289 – human<BR /> |
**[[2ld1]], [[2jm1]] - hATRX ADD domain - NMR<BR /> | **[[2ld1]], [[2jm1]] - hATRX ADD domain - NMR<BR /> | ||
| - | **[[3ql9]] – hATRX ADD domain + histone H3 peptide <BR /> | + | **[[3ql9]], [[4w5a]] – hATRX ADD domain + histone H3 peptide <BR /> |
**[[2lbm]] - hATRX ADD domain + histone H3 peptide – NMR<BR /> | **[[2lbm]] - hATRX ADD domain + histone H3 peptide – NMR<BR /> | ||
**[[3qla]], [[3qlc]] - hATRX ADD domain (mutant) + histone H3 peptide<BR /> | **[[3qla]], [[3qlc]] - hATRX ADD domain (mutant) + histone H3 peptide<BR /> | ||
| Line 154: | Line 156: | ||
**[[5fhh]] – hPIF residues 200-641<br /> | **[[5fhh]] – hPIF residues 200-641<br /> | ||
| + | **[[5o6b]], [[5o6d]] – yPIF residues 237-780 + DNA + ATP<br /> | ||
| + | **[[5o6e]] – yPIF residues 237-780 + DNA + ADP<br /> | ||
*ATP-dependent DNA helicase TA0057 | *ATP-dependent DNA helicase TA0057 | ||
**[[5h8w]] – TaTA0 + DNA – ''Thermoplasma acidophilum''<br /> | **[[5h8w]] – TaTA0 + DNA – ''Thermoplasma acidophilum''<br /> | ||
| + | |||
| + | *ATP-dependent DNA helicase LHR | ||
| + | |||
| + | **[[5v9x]] – LHR + DNA – ''Mycobacterium smegmatis''<br /> | ||
*Dead box ATP-dependent RNA helicase | *Dead box ATP-dependent RNA helicase | ||
| Line 183: | Line 191: | ||
**[[3nej]] - TtHera N terminal (mutant)<br /> | **[[3nej]] - TtHera N terminal (mutant)<br /> | ||
**[[5ivl]] – GsDB CSHA - ''Geobacillus stearothermophilus''<br /> | **[[5ivl]] – GsDB CSHA - ''Geobacillus stearothermophilus''<br /> | ||
| + | **[[5b88]] - EcDBP RRM-like domain - NMR<br /> | ||
| + | **[[5gju]] - EcDBP residues 6-210<br /> | ||
| + | **[[5gi4]] - EcDBP residues 218-445<br /> | ||
*''Dead box ATP-dependent RNA helicase complexes'' | *''Dead box ATP-dependent RNA helicase complexes'' | ||
| - | **[[3rrm]] – yDBP5 residues 91-482 (mutant) + GLE1 + NUP159 + ADP + IP6 | + | **[[3rrm]] – yDBP5 residues 91-482 (mutant) + GLE1 + NUP159 + ADP + IP6<BR /> |
**[[3rrn]] - yDBP5 residues 91-482 (mutant) + GLE1 + IP6<BR /> | **[[3rrn]] - yDBP5 residues 91-482 (mutant) + GLE1 + IP6<BR /> | ||
**[[5elx]] - yDBP5 residues 91-482 + RNA<br /> | **[[5elx]] - yDBP5 residues 91-482 + RNA<br /> | ||
| Line 211: | Line 222: | ||
**[[2j0s]], [[2j0q]], [[2hyi]] – hDDX48 + protein mago nashi homolog + RNA-binding protein 8A + protein CASC3 + RNA<BR /> | **[[2j0s]], [[2j0q]], [[2hyi]] – hDDX48 + protein mago nashi homolog + RNA-binding protein 8A + protein CASC3 + RNA<BR /> | ||
**[[2j0u]] - hDDX48 + protein CASC3<BR /> | **[[2j0u]] - hDDX48 + protein CASC3<BR /> | ||
| - | **[[2db3]] – | + | **[[2db3]] – DmVASA residues 200-623 + RNA – ''Drosophila melanogaster''<br /> |
| + | **[[5nt7]] – DmVASA RecA-like domain + maternal effect protein<br /> | ||
**[[3nbf]] - TtHera N terminal (mutant) + 8-oxo-ADP<br /> | **[[3nbf]] - TtHera N terminal (mutant) + 8-oxo-ADP<br /> | ||
**[[3moj]] - BsDBPA RNA-binding domain + RNA | **[[3moj]] - BsDBPA RNA-binding domain + RNA | ||
| Line 228: | Line 240: | ||
**[[5e3h]] - hDHX58 residues 232-925 + RNA<br /> | **[[5e3h]] - hDHX58 residues 232-925 + RNA<br /> | ||
**[[3eqt]]- hDHX58 residues 541-678 + RNA<br /> | **[[3eqt]]- hDHX58 residues 541-678 + RNA<br /> | ||
| + | **[[5vha]] – bDHX36 residues 150-1010 - bovine<br /> | ||
| + | **[[5vhc]], [[5vhd]] – bDHX36 residues 150-1010 + ADP<br /> | ||
| + | **[[5vhe]] – bDHX36 residues 56-1010 + DNA<br /> | ||
*ATP-dependent RNA helicase | *ATP-dependent RNA helicase | ||
| Line 235: | Line 250: | ||
**[[4u4c]] - yDOB1 residues 81-1073 + poly(A) RNA polymerase protein 2<br /> | **[[4u4c]] - yDOB1 residues 81-1073 + poly(A) RNA polymerase protein 2<br /> | ||
**[[4qu4]] - yDOB1 <br /> | **[[4qu4]] - yDOB1 <br /> | ||
| + | **[[5oob]] - yDOB1 residues 81-1073 + NOP53<br /> | ||
**[[5suq]] – ySUB2 + TEX1 + THO2 <br /> | **[[5suq]] – ySUB2 + TEX1 + THO2 <br /> | ||
**[[5sup]] – ySUB2 + YRA1 + RNA <br /> | **[[5sup]] – ySUB2 + YRA1 + RNA <br /> | ||
| Line 245: | Line 261: | ||
**[[1onb]], [[1jr6]] - HvHel NS3 arginine-rich domain residues 1353-1507 – NMR<BR /> | **[[1onb]], [[1jr6]] - HvHel NS3 arginine-rich domain residues 1353-1507 – NMR<BR /> | ||
**[[2jlq]], [[2bhr]], [[2bmf]], [[2whx]], [[2wzq]] – DvHel NS3 residues 1646-2092 – Dengue virus<BR /> | **[[2jlq]], [[2bhr]], [[2bmf]], [[2whx]], [[2wzq]] – DvHel NS3 residues 1646-2092 – Dengue virus<BR /> | ||
| + | **[[5xc7]] – DvHel NS3 residues 1646-2092 (mutant) <br /> | ||
**[[2z83]] – Hel NS3 residues 167-624 – Japanese encephalitis virus<BR /> | **[[2z83]] – Hel NS3 residues 167-624 – Japanese encephalitis virus<BR /> | ||
**[[2v8o]], [[2wv9]] - Hel NS3 helicase domain – Murray valley encephalitis virus<BR /> | **[[2v8o]], [[2wv9]] - Hel NS3 helicase domain – Murray valley encephalitis virus<BR /> | ||
**[[2qeq]] - Hel NS3 residues 1691-2124 – Kunjin virus<BR /> | **[[2qeq]] - Hel NS3 residues 1691-2124 – Kunjin virus<BR /> | ||
| - | **[[5txg]], [[5jmt]] - ZvHel NS3 | + | **[[5txg]], [[5jmt]], [[5vi7]], [[5jps]] - ZvHel NS3 helicase domain – Zika virus<br /> |
**[[5k8u]], [[5k8l]], [[5k8i]], [[5jwh]], [[5jrz]] - ZvHel NS3 residues 164-621<br /> | **[[5k8u]], [[5k8l]], [[5k8i]], [[5jwh]], [[5jrz]] - ZvHel NS3 residues 164-621<br /> | ||
| + | **[[5x8y]] - ZvHel NS1 residues 168-352<br /> | ||
**[[2v6i]] – KvHel helicase domain – Kokobera virus<BR /> | **[[2v6i]] – KvHel helicase domain – Kokobera virus<BR /> | ||
| - | **[[2v6j]] - KvHel helicase domain (mutant) | + | **[[2v6j]] - KvHel helicase domain (mutant)<br /> |
| + | **[[5wso]], [[5gvu]] - Hel NS3 residues 1939-2414 – bovine viral diarrhea virus<br /> | ||
*''Viral ATP-dependent RNA helicase NS3 complexes'' | *''Viral ATP-dependent RNA helicase NS3 complexes'' | ||
| Line 261: | Line 280: | ||
**[[2jlr]] - DvHel NS3 residues 1646-2092 + AMPPNP<BR /> | **[[2jlr]] - DvHel NS3 residues 1646-2092 + AMPPNP<BR /> | ||
**[[2jls]] - DvHel NS3 residues 1646-2092 + ADP<BR /> | **[[2jls]] - DvHel NS3 residues 1646-2092 + ADP<BR /> | ||
| - | **[[2jlu]], [[2jlw]] - DvHel NS3 residues 1646-2092 + RNA<BR /> | + | **[[2jlu]], [[2jlw]], [[5xc6]] - DvHel NS3 residues 1646-2092 + RNA<BR /> |
**[[2jlv]] - DvHel NS3 residues 1646-2092 + AMPPNP + RNA<BR /> | **[[2jlv]] - DvHel NS3 residues 1646-2092 + AMPPNP + RNA<BR /> | ||
**[[2jlx]], [[2jly]], [[2jlz]] - DvHel NS3 residues 1646-2092 + ADP + RNA<BR /> | **[[2jlx]], [[2jly]], [[2jlz]] - DvHel NS3 residues 1646-2092 + ADP + RNA<BR /> | ||
| Line 268: | Line 287: | ||
**[[5gjc]] - ZvHel NS3 residues 1676-2119 + ATP<br /> | **[[5gjc]] - ZvHel NS3 residues 1676-2119 + ATP<br /> | ||
**[[5gjb]] - ZvHel NS3 residues 1676-2119 + RNA<br /> | **[[5gjb]] - ZvHel NS3 residues 1676-2119 + RNA<br /> | ||
| + | **[[5y4z]] - ZvHel NS3 residues 1676-2119 + AMPPNP<br /> | ||
*ATP-dependent RNA helicase SUV3 | *ATP-dependent RNA helicase SUV3 | ||
| Line 274: | Line 294: | ||
**[[3og8]] – hDDX58 C terminal + RNA<BR /> | **[[3og8]] – hDDX58 C terminal + RNA<BR /> | ||
**[[3rc8]] - hSUV3 residues 47-722 + RNA<BR /> | **[[3rc8]] - hSUV3 residues 47-722 + RNA<BR /> | ||
| + | **[[6f4a]] – ySUV3 + exoribonuclease II + RNA<br /> | ||
*ATP-dependent RNA helicase A | *ATP-dependent RNA helicase A | ||
| Line 312: | Line 333: | ||
**[[2v1x]] - hQ1 residues 49-616<BR /> | **[[2v1x]] - hQ1 residues 49-616<BR /> | ||
**[[2wwy]], [[4u7d]] – hQ1 residues 49-616 + DNA<br /> | **[[2wwy]], [[4u7d]] – hQ1 residues 49-616 + DNA<br /> | ||
| + | **[[5lst]] – hQ4 residues 427-1116<br /> | ||
**[[2kmu]] – hQ4 N terminal - NMR<br /> | **[[2kmu]] – hQ4 N terminal - NMR<br /> | ||
**[[5lb8]] – hQ5 <br /> | **[[5lb8]] – hQ5 <br /> | ||
| Line 346: | Line 368: | ||
**[[1in4]] – TmRuvB<BR /> | **[[1in4]] – TmRuvB<BR /> | ||
**[[1in5]], [[1in6]], [[1in7]], [[1in8]], [[1j7k]] – TmRuvB (mutant)<br /> | **[[1in5]], [[1in6]], [[1in7]], [[1in8]], [[1j7k]] – TmRuvB (mutant)<br /> | ||
| - | **[[3pfi]] – RuvB + ADP – ''Campylobacter jejuni'' | + | **[[3pfi]] – RuvB + ADP – ''Campylobacter jejuni''<br /> |
| + | **[[6blb]] - PaRuvB + ADP - ''Pseudomonas aeruginosa''<br /> | ||
*ATP-dependent DNA helicase UVSW | *ATP-dependent DNA helicase UVSW | ||
| Line 446: | Line 469: | ||
**[[4qqy]] – TfCas3 + ADP + HD nuclease<br /> | **[[4qqy]] – TfCas3 + ADP + HD nuclease<br /> | ||
**[[4qqz]] – TfCas3 + AMPPNP <br /> | **[[4qqz]] – TfCas3 + AMPPNP <br /> | ||
| - | **[[5gqh]] – PaCas3 + anti-crispr protein 3 | + | **[[5gqh]] – PaCas3 + anti-crispr protein 3 <br /> |
**[[5b7i]] – PaCas3 + ACRF3<br /> | **[[5b7i]] – PaCas3 + ACRF3<br /> | ||
Revision as of 08:12, 20 June 2018
| |||||||||||
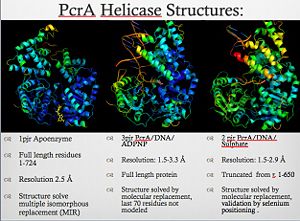
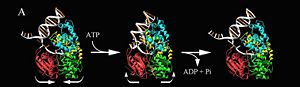
3D structures of helicase
Updated on 20-June-2018
References
Crystal structure of a DExx box DNA helicase., Subramanya HS, Bird LE, Brannigan JA, Wigley DB, Nature. 1996 Nov 28;384(6607):379-83. PMID:8934527
^ Johnson DS, Bai L, Smith BY, Patel SS, Wang MD (2007). "Single-molecule studies reveal dynamics of DNA unwinding by the ring-shaped t7 helicase". Cell 129 (7): 1299–309. doi:10.1016/j.cell.2007.04.038. PMID 17604719.
^ a b "Researchers solve mystery of how DNA strands separate" (2007-07-03). Retrieved on 2007-07-05.
^ Dumont S, Cheng W, Serebrov V, Beran RK, Tinoco Jr I, Pylr AM, Bustamante C, "RNA Translocation and Unwinding Mechanism of HCV NS3 Helicase and its Coordination by ATP", Nature. 2006 Jan 5; 439: 105-108.
Anand SP, Zheng H, Bianco PR, Leuba SH, Khan SA. DNA helicase activity of PcrA is not required for displacement of RecA protein from DNA or inhibition of RecA-mediated DNA strand exchange. Journal of Bacteriology (2007) 189 (12):4502-4509.
Bird L, Subramanya HS, Wigley DB, "Helicases: a unifying structural theme?", Current Opinion in Structural Biology. 1998 Feb; 8 (1): 14-18.
Betterton MD, Julicher F, "Opening of nucleic-acid double strands by helicases: active versus passive opening.", Physical Review E. 2005 Jan; 71 (1): 011904.
- Sengoku T, Nureki O, Nakamura A, Kobayashi S, Yokoyama S. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell. 2006 Apr 21;125(2):287-300. PMID:16630817 doi:10.1016/j.cell.2006.01.054
- Sengoku T, Nureki O, Dohmae N, Nakamura A, Yokoyama S. Crystallization and preliminary X-ray analysis of the helicase domains of Vasa complexed with RNA and an ATP analogue. Acta Crystallogr D Biol Crystallogr. 2004 Feb;60(Pt 2):320-2. Epub 2004, Jan 23. PMID:14747711 doi:10.1107/S0907444903025897
Content Donators
Created with the participation of Luis E Ramirez-Tapia, Wayne Decatur.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Wayne Decatur, Joel L. Sussman