Sandbox Reserved 1508
From Proteopedia
| Line 7: | Line 7: | ||
| - | == | + | == Background== |
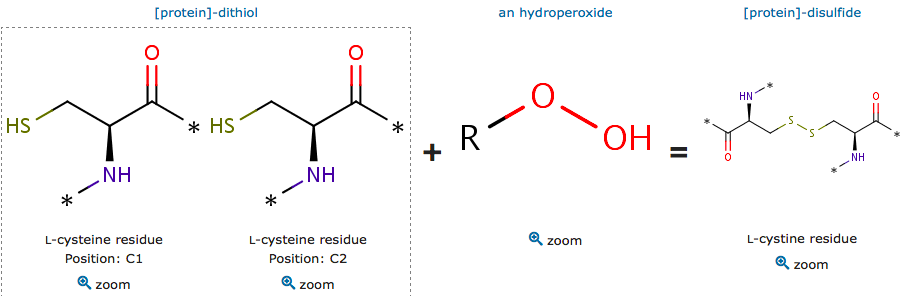
| - | + | In oxidative stress, the organism manage to do a reduction of peroxides. This reaction is catalyzed by the peroxiredoxins. From a structural point of view, a specific amino acid is involved in this reaction: its a nucleophilic cystein, called peroxidatic cystein. In order to understand the mechanism and the specificity of this reaction according to its specific chemical environment, researchers used the ''Mycobacterium tuberculosis'' alkyl hydroperoxide reductase E (MtAhpE) as model (Pedre et al, 2016). The mutational effects of key residues in its environment are located in the active site. These amino acids create an environment favorising the reaction with peroxides. | |
Peroxiredoxins are peroxidases which catalyze the reduction of peroxides (organic peroxide H2O2 or organic hydroperoxides).</div> | Peroxiredoxins are peroxidases which catalyze the reduction of peroxides (organic peroxide H2O2 or organic hydroperoxides).</div> | ||
| Line 22: | Line 22: | ||
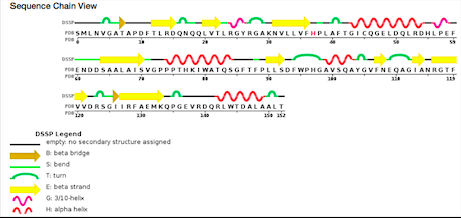
According to PDB code 5C04, the structure of the protein has been characterized thanks to crystallography (X-Ray diffraction). The scientists obtained a resolution of 1.45 Å, a R-value free of 0.198 and a R-value work of 0.166. The structure of the 5C04 protein has in total 2 chains (A and B) represented by one sequence-unique entity (one polymer) of L-type polypeptide. Its length is 153 residues. Its secondary structure shows that 27% of alpha helix are composed by six helix for a total of 42 residues; and 27% of beta sheet are composed by 11 strands for also a total of 42 residues. | According to PDB code 5C04, the structure of the protein has been characterized thanks to crystallography (X-Ray diffraction). The scientists obtained a resolution of 1.45 Å, a R-value free of 0.198 and a R-value work of 0.166. The structure of the 5C04 protein has in total 2 chains (A and B) represented by one sequence-unique entity (one polymer) of L-type polypeptide. Its length is 153 residues. Its secondary structure shows that 27% of alpha helix are composed by six helix for a total of 42 residues; and 27% of beta sheet are composed by 11 strands for also a total of 42 residues. | ||
[[Image:sequence.png]] | [[Image:sequence.png]] | ||
| - | ''Sequence of the 5C04 protein'' | + | ''Sequence of the 5C04 protein'' |
| - | [[Image: | + | [[Image:seq2.png]] |
| - | ''Sequence view chain'' | + | ''Sequence view chain'' |
== Catalytic site == | == Catalytic site == | ||
Revision as of 14:25, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
The protein 5C04
| |||||||||||
References
Ågren, Daniel, Robert Schnell, Wulf Oehlmann, Mahavir Singh, et Gunter Schneider. « Cysteine Synthase (CysM) of Mycobacterium Tuberculosis Is an O -Phosphoserine Sulfhydrylase: EVIDENCE FOR AN ALTERNATIVE CYSTEINE BIOSYNTHESIS PATHWAY IN MYCOBACTERIA ». Journal of Biological Chemistry 283, nᵒ 46 (14 novembre 2008): 31567‑74. https://doi.org/10.1074/jbc.M804877200.
Burns, Kristin E., Sabine Baumgart, Pieter C. Dorrestein, Huili Zhai, Fred W. McLafferty, et Tadhg P. Begley. « Reconstitution of a New Cysteine Biosynthetic Pathway in Mycobacterium t Uberculosis ». Journal of the American Chemical Society 127, nᵒ 33 (août 2005): 11602‑3. https://doi.org/10.1021/ja053476x.
Pedre, Brandán, Laura A. H. van Bergen, Anna Palló, Leonardo A. Rosado, Veronica Tamu Dufe, Inge Van Molle, Khadija Wahni, et al. « The Active Site Architecture in Peroxiredoxins: A Case Study on Mycobacterium Tuberculosis AhpE ». Chemical Communications 52, nᵒ 67 (2016): 10293‑96. https://doi.org/10.1039/C6CC02645A.
Rhee, Sue Goo, et Hyun Ae Woo. « Multiple Functions of Peroxiredoxins: Peroxidases, Sensors and Regulators of the Intracellular Messenger H 2 O 2 , and Protein Chaperones ». Antioxidants & Redox Signaling 15, nᵒ 3 (août 2011): 781‑94. https://doi.org/10.1089/ars.2010.3393.
Zeida, Ari, Aníbal M. Reyes, Pablo Lichtig, Martín Hugo, Diego S. Vazquez, Javier Santos, F. Luis González Flecha, Rafael Radi, Dario A. Estrin, et Madia Trujillo. « Molecular Basis of Hydroperoxide Specificity in Peroxiredoxins: The Case of AhpE from Mycobacterium Tuberculosis ». Biochemistry 54, nᵒ 49 (15 décembre 2015): 7237‑47. https://doi.org/10.1021/acs.biochem.5b00758.