User:Karsten Theis/Insulin
From Proteopedia
(→Structure) |
|||
| Line 41: | Line 41: | ||
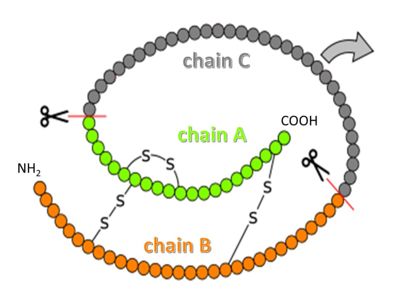
| - | <scene name='82/821037/Proinsulin/1'>Proinsulin</scene> (Pins) is processed by several proteases in the Golgi apparatus to form 3 separate chains, named B, C and A (see figure above)<ref>PMID:15289650</ref>. Chains B and A are linked through disulfide bonds, and are the components of mature insulin. Chain C, flexible and somewhat disordered in the proinsulin structure, is an independent peptide whose biological role after processing is unclear. | + | <scene name='82/821037/Proinsulin/1'>Proinsulin</scene> (Pins) is processed by several proteases in the Golgi apparatus to form 3 separate chains, named B, C and A (see figure above)<ref>PMID:15289650</ref>. Chains B and A are linked through disulfide bonds, and are the components of mature insulin. |
| + | |||
| + | <jmol> | ||
| + | <jmolRadioGroup> | ||
| + | <item> | ||
| + | <script> select select (29-64); backbone off </script> | ||
| + | <text>mature insulin</text> | ||
| + | <checked>false</checked> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script> select select (29-64); backbone on </script> | ||
| + | <text>proinsulin</text> | ||
| + | <checked>false</checked> | ||
| + | </item> | ||
| + | </jmolRadioGroup> | ||
| + | </jmol> | ||
| + | |||
| + | |||
| + | Chain C, flexible and somewhat <jmol> | ||
| + | <jmolLink> | ||
| + | <script>anim mode loop; anim on</script> | ||
| + | <text>disordered</text></jmolLink></jmol> in the proinsulin structure, is an independent peptide whose biological role after processing is unclear. | ||
Before insulin is secreted, it is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains. These <scene name='User:Whitney_Stoppel/sandbox1/Insulin_dimer/2'>hydrogen bonds</scene> are shown above in white. Then, 3 dimers can come together in the presence of zinc ions and form a hexamer. Insulin is stored in the <scene name='User:Whitney_Stoppel/sandbox1/Insulin_hexamer/4'>hexameric form</scene> in the body. This <scene name='User:Whitney_Stoppel/sandbox1/Insulin_ph7/2'>scene highlights</scene> the hydrophobic (gray) and polar (purple) parts of an insulin monomer at a pH of 7. | Before insulin is secreted, it is able to pair-up with itself and form a dimer by forming hydrogen bonds between the ends of two B-chains. These <scene name='User:Whitney_Stoppel/sandbox1/Insulin_dimer/2'>hydrogen bonds</scene> are shown above in white. Then, 3 dimers can come together in the presence of zinc ions and form a hexamer. Insulin is stored in the <scene name='User:Whitney_Stoppel/sandbox1/Insulin_hexamer/4'>hexameric form</scene> in the body. This <scene name='User:Whitney_Stoppel/sandbox1/Insulin_ph7/2'>scene highlights</scene> the hydrophobic (gray) and polar (purple) parts of an insulin monomer at a pH of 7. | ||
Revision as of 19:48, 11 July 2019
Insulin is a peptide hormone that controls carbohydrate metabolism and storage in the human body[1][2]. It is secreted by specialized cells in the pancreas, enters the bloodstream and reaches other cells. There, it binds to the extracellular side of the insulin receptor, triggering tyrosine kinase activity within the target cell, which in turn regulates glucose uptake, metabolism and storage.
Contents |
Function
The body is able to sense the concentration of glucose in the blood and respond by secreting insulin, which is produced by beta cells in the pancreas. Insulin then binds to the insulin receptor, changing its conformation [3]. Depending on cell type and the presence of other signals (glucogon, epinephrine), the cell will modify its metabolism.
Disease
In patients with diabetes, insulin signalling is compromised[4]. Synthesis of human insulin in E. coli is important to producing insulin for the treatment of type 1 diabetes. It is believed that the hydrophobic sections on the B-chain cause insulin aggregation which initially caused problems in the manufacture and storage of insulin for pharmaceutical use.
Structure
| |||||||||||
References
- ↑ Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000 Jul;85(1):69-79. PMID:10927996
- ↑ Weiss MA, Lawrence MC. A thing of beauty: Structure and function of insulin's "aromatic triplet". Diabetes Obes Metab. 2018 Sep;20 Suppl 2:51-63. doi: 10.1111/dom.13402. PMID:30230175 doi:http://dx.doi.org/10.1111/dom.13402
- ↑ Gutmann T, Kim KH, Grzybek M, Walz T, Coskun U. Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor. J Cell Biol. 2018 May 7;217(5):1643-1649. doi: 10.1083/jcb.201711047. Epub 2018, Feb 16. PMID:29453311 doi:http://dx.doi.org/10.1083/jcb.201711047
- ↑ https://www.endotext.org/section/diabetes/
- ↑ Davidson HW. (Pro)Insulin processing: a historical perspective. Cell Biochem Biophys. 2004;40(3 Suppl):143-58. PMID:15289650