We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Isocitrate dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
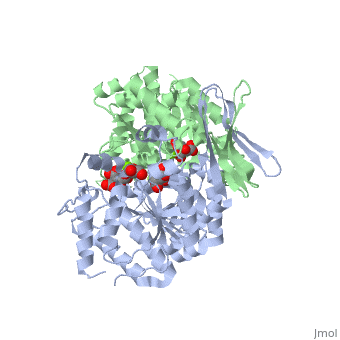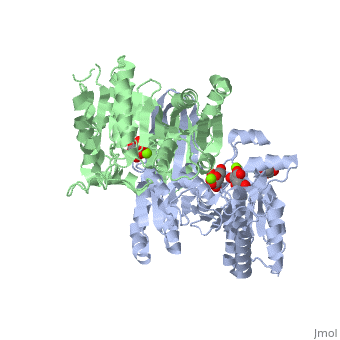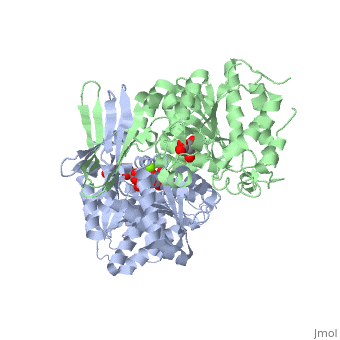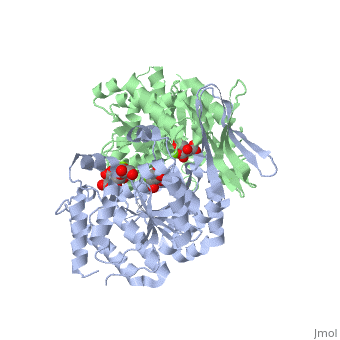
<StructureSection load='2uxr' size='350' side='right' scene='' caption='Isocitrate dehydrogenase dimer complex with isocitrate, glycerol and Mg+2 ion (green) (PDB code [[2uxr]])'> | <StructureSection load='2uxr' size='350' side='right' scene='' caption='Isocitrate dehydrogenase dimer complex with isocitrate, glycerol and Mg+2 ion (green) (PDB code [[2uxr]])'> | ||
==Introduction== | ==Introduction== | ||
| - | [[Isocitrate dehydrogenase]] (IDH) is an enzyme that is used during the third step of the [[Citric Acid Cycle|citric acid cycle]]. This biological reaction is an essential process that is used to create molecules that are used for cellular energy. In this step it catalyzes the oxidative decarboxylation of isocitrate meaning that CO2 is released from the isocitrate. In addition coenzyme [http://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NAD]+ is converted to an [http://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NADH]. This reaction results in an alpha-ketoglutarate molecule which is then moved on to the forth step of the citric acid cycle. In ''Escherichia coli'' the IDH is regulated by phosphorylation which is catalyzed by [[Isocitrate dehydrogenase kinase/phosphatase|IDH kinase/phosphatase (IDHK/P)]]. See also:<br /> | + | [[Isocitrate dehydrogenase]] (IDH) is an enzyme that is used during the third step of the [[Citric Acid Cycle|citric acid cycle]]. This biological reaction is an essential process that is used to create molecules that are used for cellular energy. In this step it catalyzes the oxidative decarboxylation of isocitrate meaning that CO2 is released from the isocitrate. In addition coenzyme [http://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NAD]+ is converted to an [http://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NADH]. This reaction results in an alpha-ketoglutarate molecule which is then moved on to the forth step of the citric acid cycle. In ''Escherichia coli'' the IDH is regulated by phosphorylation which is catalyzed by [[Isocitrate dehydrogenase kinase/phosphatase|IDH kinase/phosphatase (IDHK/P)]]. Human '''IDH1''' is cytoplasmic while '''IDH2''' is mitochondrial. See also:<br /> |
*[[Krebs cycle carbons]] | *[[Krebs cycle carbons]] | ||
*[[Krebs cycle importance]] | *[[Krebs cycle importance]] | ||
Current revision
| |||||||||||
References
- ↑ Xu X, Zhao J, Xu Z, Peng B, Huang Q, Arnold E, Ding J. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem. 2004 Aug 6;279(32):33946-57. Epub 2004 Jun 1. PMID:15173171 doi:10.1074/jbc.M404298200
- ↑ Fedoy AE, Yang N, Martinez A, Leiros HK, Steen IH. Structural and functional properties of isocitrate dehydrogenase from the psychrophilic bacterium Desulfotalea psychrophila reveal a cold-active enzyme with an unusual high thermal stability. J Mol Biol. 2007 Sep 7;372(1):130-49. Epub 2007 Jun 19. PMID:17632124 doi:10.1016/j.jmb.2007.06.040
- ↑ http://en.wikipedia.org/wiki/Isocitrate_dehydrogenase#cite_note-nfr154197.2F32-6
- ↑ Xu X, Zhao J, Xu Z, Peng B, Huang Q, Arnold E, Ding J. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem. 2004 Aug 6;279(32):33946-57. Epub 2004 Jun 1. PMID:15173171 doi:10.1074/jbc.M404298200
- ↑ Xu X, Zhao J, Xu Z, Peng B, Huang Q, Arnold E, Ding J. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem. 2004 Aug 6;279(32):33946-57. Epub 2004 Jun 1. PMID:15173171 doi:10.1074/jbc.M404298200
- ↑ http://en.wikipedia.org/wiki/File:IDHcatalyticmechanism.jpg
- ↑ Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, Tallman MS, Sun Z, Wolniak K, Peeters JK, Liu W, Choe SE, Fantin VR, Paietta E, Lowenberg B, Licht JD, Godley LA, Delwel R, Valk PJ, Thompson CB, Levine RL, Melnick A. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010 Dec 14;18(6):553-67. Epub 2010 Dec 9. PMID:21130701 doi:10.1016/j.ccr.2010.11.015
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Michael Nobbe, Joel L. Sussman