Main Page
From Proteopedia
(Undo revision 3114449 by Deja Sanders (Talk)) |
|||
| Line 1: | Line 1: | ||
| - | <table id=" | + | <table id="tableColumnsMainPage" style="width:100%;border:2px solid #ddd;border-collapse: collapse;table-layout: fixed; "> |
<tr><td colspan='3' style="background:#F5F5FC;border:1px solid #ddd;"> | <tr><td colspan='3' style="background:#F5F5FC;border:1px solid #ddd;"> | ||
<div style="top:+0.2em; font-size:1.2em; padding:5px 5px 5px 10px; float:right;">'''''ISSN 2310-6301'''''</div> | <div style="top:+0.2em; font-size:1.2em; padding:5px 5px 5px 10px; float:right;">'''''ISSN 2310-6301'''''</div> | ||
Revision as of 05:43, 26 November 2019
|
ISSN 2310-6301
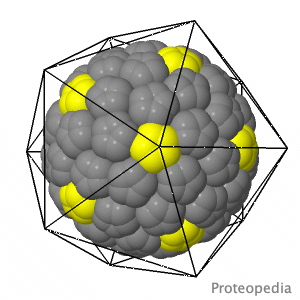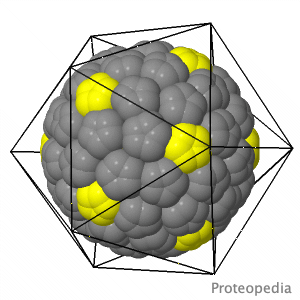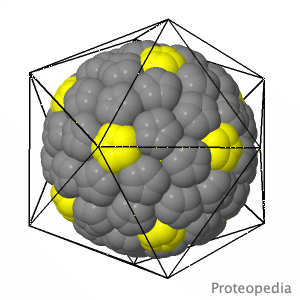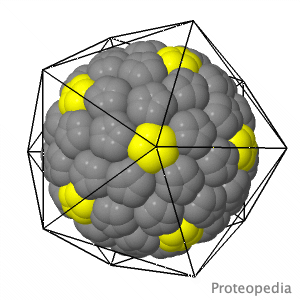
As life is more than 2D, Proteopedia helps to bridge the gap between 3D structure & function of biomacromolecules Proteopedia presents this information in a user-friendly way as a collaborative & free 3D-encyclopedia of proteins & other biomolecules.
| ||||||||
| Selected Research Pages | In Journals | Education | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
||||||
|
How to add content to Proteopedia Who knows ... |
Teaching strategies using Proteopedia |
|||||||
| ||||||||