We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1613
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
=''ABCG2 Transporter Protein''= | =''ABCG2 Transporter Protein''= | ||
| - | <StructureSection | + | <StructureSection load='6ffc' size='350' frame='true' side='right' caption='ABCG2 6ffc' scene=’’> |
==Introduction== | ==Introduction== | ||
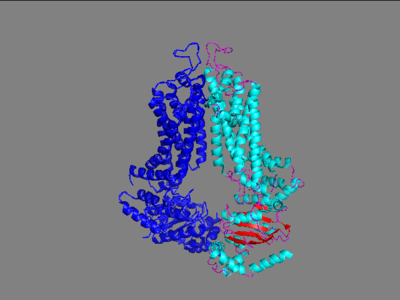
The ABCG2 transporter protein is a notable transmembrane protein that transports xenobiotic material out of numerous cells especially those located on the blood-brain barrier. ABCG2 belongs to the family of 48 transporter proteins called ATP-binding cassette transporters (ABC transporters) that are made unique from each other by their size, structure and their ordering of specific domains. This family has been found as a prevalent piece of multi-drug resistant cancers and therefore became a popular target towards inhibition. Three generations of drugs were made in order to inhibit a similar protein from the same family, ABCC1 at its interior binding site including cyclosporine A (first generation), valspodar (second generation), and Elacridar (3rd generation). Importantly, cyclosporine A and Elacridar were found to inhibit both ABCC1 and ABCG2 and in one trial had success along with chemotherapy in the treatment of acute myeloid leukemia but because of either side effects or experimentation that was not able to be duplicated, this research was mostly shelved. The main issue in their failure to find a drug to inhibit this protein was the failure to develop a high-resolution structure of this protein with the technology available at the time of this drug development. In the mid 2010's, upgrades to cryo-electron microscopy and the use of 2 antigen binding fragments (5D3-Fab) allowed for high resolution images to finally be developed for ABCG2 transporter protein. With these recent discoveries, the understanding of this protein has greatly increased in the last several years. | The ABCG2 transporter protein is a notable transmembrane protein that transports xenobiotic material out of numerous cells especially those located on the blood-brain barrier. ABCG2 belongs to the family of 48 transporter proteins called ATP-binding cassette transporters (ABC transporters) that are made unique from each other by their size, structure and their ordering of specific domains. This family has been found as a prevalent piece of multi-drug resistant cancers and therefore became a popular target towards inhibition. Three generations of drugs were made in order to inhibit a similar protein from the same family, ABCC1 at its interior binding site including cyclosporine A (first generation), valspodar (second generation), and Elacridar (3rd generation). Importantly, cyclosporine A and Elacridar were found to inhibit both ABCC1 and ABCG2 and in one trial had success along with chemotherapy in the treatment of acute myeloid leukemia but because of either side effects or experimentation that was not able to be duplicated, this research was mostly shelved. The main issue in their failure to find a drug to inhibit this protein was the failure to develop a high-resolution structure of this protein with the technology available at the time of this drug development. In the mid 2010's, upgrades to cryo-electron microscopy and the use of 2 antigen binding fragments (5D3-Fab) allowed for high resolution images to finally be developed for ABCG2 transporter protein. With these recent discoveries, the understanding of this protein has greatly increased in the last several years. | ||
Revision as of 00:17, 31 March 2020
ABCG2 Transporter Protein
| |||||||||||
References
- ↑ Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
- ↑ Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
Student Contributors
Samuel Sullivan, Jaelyn Voyles, Shelby Skaggs