We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1613
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
==General Structure== | ==General Structure== | ||
The ABCG2 protein is comprised of a homodimer which each have two specific domains: one spanning the cell membrane and one involved with nucleotide binding. | The ABCG2 protein is comprised of a homodimer which each have two specific domains: one spanning the cell membrane and one involved with nucleotide binding. | ||
| + | === Structural highlights === | ||
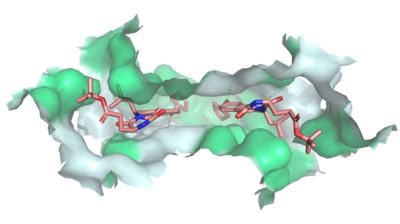
| + | [[Image:Ligand_Interactions_6ffc.png|400 px|right|thumb|Figure 2]] | ||
| + | |||
| + | Multidrug Transporter ABCG2 is a <scene name='83/832937/Dimer/1'>dimer</scene> that consists of two cavities seperated by a <scene name='83/832937/Leucine_plug/3'>leucine plug</scene>. Cavity 1 is a binding pocket open to the cytoplasm and the inner leaflet of the plasma membrane. Its shape is suitable to bind flat, hydrophobic and polycyclic substrates. Many of its amino acids residues form hydrophobic interactions with the bound substrate, as shown in green in '''Figure 1'''. Cavity 2 is located above the leucine plug. It is empty until a <scene name='83/832937/Atp_and_mg_bound_to_abcg2/3'>magnesium ion and ATP</scene> are bound to ABCG2. Its <scene name='83/832937/Cysteine_disulfide_bridges/4'>inter- and intra-disulfides</scene> (red is inter- and intra-molecular disulfides, purple is intra-molecular only) promote the release of the substrate from the cavity into the extracellular space. | ||
| + | <ref name=”Jackson”>PMID:29610494</ref> | ||
| + | <ref name="Manolaridis">PMID:30405239</ref> | ||
===Transmembrane Domains=== | ===Transmembrane Domains=== | ||
In the transmembrane domain is located the leucine plug that separates the first site of binding from the second site of binding for the substrate. This is only opened when the protein is shifted from its interior facing to exterior facing formation by the transfer of a phosphate off of an ATP. This causes a shift and transfer to the second binding site which is open to the extracellular matrix. The Fab-5D3 antigen binding fragment was found to stabilize the protein in its inward facing conformation which allowed for high resolution images of the protein to be taken in this conformation. Without Fab attached, the conformation of the protein was constantly in flux, transporting substrates out of the cell constantly. Fab was found to only stabilize ABCG2 on the extracellular side. Three important interactions between Fab fragments and ABCG2 were important to binding stability and favorability. This binding occurred at the <scene name='83/832939/El-3/1'>EL-3</scene> of ABCG2 which is the helices that stretches the furthest from the cell membrane. These interactions were two disulfide bonds, one intramolecularly and the other intermolecularly, and an n-<scene name='83/832939/Glycos_site/1'>glycosylation site</scene> at residue Asn-596. | In the transmembrane domain is located the leucine plug that separates the first site of binding from the second site of binding for the substrate. This is only opened when the protein is shifted from its interior facing to exterior facing formation by the transfer of a phosphate off of an ATP. This causes a shift and transfer to the second binding site which is open to the extracellular matrix. The Fab-5D3 antigen binding fragment was found to stabilize the protein in its inward facing conformation which allowed for high resolution images of the protein to be taken in this conformation. Without Fab attached, the conformation of the protein was constantly in flux, transporting substrates out of the cell constantly. Fab was found to only stabilize ABCG2 on the extracellular side. Three important interactions between Fab fragments and ABCG2 were important to binding stability and favorability. This binding occurred at the <scene name='83/832939/El-3/1'>EL-3</scene> of ABCG2 which is the helices that stretches the furthest from the cell membrane. These interactions were two disulfide bonds, one intramolecularly and the other intermolecularly, and an n-<scene name='83/832939/Glycos_site/1'>glycosylation site</scene> at residue Asn-596. | ||
| Line 19: | Line 25: | ||
One of the causes for multidrug resistant cancers is the excretion of cancer drugs out of the cell, thereby decreasing the effective intracellular concentration. ABCG2, also known as the breast cancer resistance protein (BCRP), effluxes multiple chemotherapeutic agents such as [https://en.wikipedia.org/wiki/Mitoxantrone mitoxantrone] and [https://en.wikipedia.org/wiki/Camptothecin camptothecin] analogies, making the cancerous breast cells resistant to chemotherapy. Competitive inhibitors, such as <scene name='83/832939/Abcg2_bound_to_mz29/3'>MZ29</scene>, that shut down ABCG2 to stop the efflux of cancer drugs in order to combat the resistivity of breast cancer. <ref>[ https://en.wikipedia.org/wiki/ABCG2 "ABCG2 -." Wikipedia, the Free Encyclopedia. Web. 20 Apr. 2020].</ref><ref name=”Jackson”>PMID:29610494</ref> | One of the causes for multidrug resistant cancers is the excretion of cancer drugs out of the cell, thereby decreasing the effective intracellular concentration. ABCG2, also known as the breast cancer resistance protein (BCRP), effluxes multiple chemotherapeutic agents such as [https://en.wikipedia.org/wiki/Mitoxantrone mitoxantrone] and [https://en.wikipedia.org/wiki/Camptothecin camptothecin] analogies, making the cancerous breast cells resistant to chemotherapy. Competitive inhibitors, such as <scene name='83/832939/Abcg2_bound_to_mz29/3'>MZ29</scene>, that shut down ABCG2 to stop the efflux of cancer drugs in order to combat the resistivity of breast cancer. <ref>[ https://en.wikipedia.org/wiki/ABCG2 "ABCG2 -." Wikipedia, the Free Encyclopedia. Web. 20 Apr. 2020].</ref><ref name=”Jackson”>PMID:29610494</ref> | ||
| - | == Structural highlights == | ||
| - | [[Image:Ligand_Interactions_6ffc.png|400 px|right|thumb|Figure 2]] | ||
| - | |||
| - | Multidrug Transporter ABCG2 is a <scene name='83/832937/Dimer/1'>dimer</scene> that consists of two cavities seperated by a <scene name='83/832937/Leucine_plug/3'>leucine plug</scene>. Cavity 1 is a binding pocket open to the cytoplasm and the inner leaflet of the plasma membrane. Its shape is suitable to bind flat, hydrophobic and polycyclic substrates. Many of its amino acids residues form hydrophobic interactions with the bound substrate, as shown in green in '''Figure 1'''. Cavity 2 is located above the leucine plug. It is empty until a <scene name='83/832937/Atp_and_mg_bound_to_abcg2/3'>magnesium ion and ATP</scene> are bound to ABCG2. Its <scene name='83/832937/Cysteine_disulfide_bridges/4'>inter- and intra-disulfides</scene> (red is inter- and intra-molecular disulfides, purple is intra-molecular only) promote the release of the substrate from the cavity into the extracellular space. | ||
| - | <ref name=”Jackson”>PMID:29610494</ref> | ||
| - | <ref name="Manolaridis">PMID:30405239</ref> | ||
</StructureSection> | </StructureSection> | ||
Revision as of 04:22, 21 April 2020
ABCG2 Transporter Protein
| |||||||||||
References
- ↑ Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
- ↑ Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, Bates SE. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006 Apr 8;235(1):84-92. doi: 10.1016/j.canlet.2005.04.024. Epub, 2005 Jun 28. PMID:15990223 doi:http://dx.doi.org/10.1016/j.canlet.2005.04.024
- ↑ Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
- ↑ Cleophas MC, Joosten LA, Stamp LK, Dalbeth N, Woodward OM, Merriman TR. ABCG2 polymorphisms in gout: insights into disease susceptibility and treatment approaches. Pharmgenomics Pers Med. 2017 Apr 20;10:129-142. doi: 10.2147/PGPM.S105854., eCollection 2017. PMID:28461764 doi:http://dx.doi.org/10.2147/PGPM.S105854
- ↑ [ https://en.wikipedia.org/wiki/ABCG2 "ABCG2 -." Wikipedia, the Free Encyclopedia. Web. 20 Apr. 2020].
- ↑ Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
Student Contributors
Shelby Skaggs, Samuel Sullivan, Jaelyn Voyles