Sandbox Reserved 1613
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
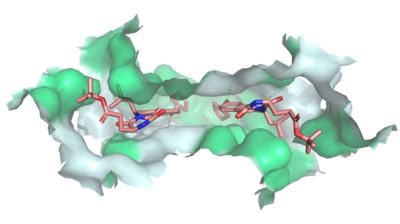
[[Image:Ligand_Interactions_6ffc.png|400 px|right|thumb|Figure 1: MZ29 bound to cavity 1 of ABCG2 (6ffc). Two MZ29 are shown in sticks and are colored by element. Hydrophobic interactions between the surface of cavity 1 and MZ29 are shown in green.]] | [[Image:Ligand_Interactions_6ffc.png|400 px|right|thumb|Figure 1: MZ29 bound to cavity 1 of ABCG2 (6ffc). Two MZ29 are shown in sticks and are colored by element. Hydrophobic interactions between the surface of cavity 1 and MZ29 are shown in green.]] | ||
| - | Multidrug Transporter ABCG2 is a <scene name='83/832937/Dimer/1'>dimer</scene> that consists of two cavities seperated by a <scene name='83/832937/Leucine_plug/4'>leucine plug</scene>. Cavity 1 is a binding pocket open to the cytoplasm and the inner leaflet of the plasma membrane. Its shape is suitable to bind flat, hydrophobic and polycyclic substrates. Many of its amino acids residues form hydrophobic interactions with the bound substrate, as shown in green in '''Figure 1'''. Cavity 2 is located above the leucine plug. It is empty until a <scene name='83/832937/Atp_and_mg_bound_to_abcg2/ | + | Multidrug Transporter ABCG2 is a <scene name='83/832937/Dimer/1'>dimer</scene> that consists of two cavities seperated by a <scene name='83/832937/Leucine_plug/4'>leucine plug</scene>. Cavity 1 is a binding pocket open to the cytoplasm and the inner leaflet of the plasma membrane. Its shape is suitable to bind flat, hydrophobic and polycyclic substrates. Many of its amino acids residues form hydrophobic interactions with the bound substrate, as shown in green in '''Figure 1'''. Cavity 2 is located above the leucine plug. It is empty until a <scene name='83/832937/Atp_and_mg_bound_to_abcg2/4'>magnesium ion and ATP</scene> are bound to ABCG2. Its <scene name='83/832937/Cysteine_disulfide_bridges/4'>inter- and intra-disulfides</scene> (red is inter- and intra-molecular disulfides, purple is intra-molecular only) promote the release of the substrate from the cavity into the extracellular space. |
<ref name=”Jackson”>PMID:29610494</ref> | <ref name=”Jackson”>PMID:29610494</ref> | ||
<ref name="Manolaridis">PMID:30405239</ref> | <ref name="Manolaridis">PMID:30405239</ref> | ||
Revision as of 05:17, 21 April 2020
ABCG2 Transporter Protein
| |||||||||||
References
- ↑ Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
- ↑ Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, Bates SE. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006 Apr 8;235(1):84-92. doi: 10.1016/j.canlet.2005.04.024. Epub, 2005 Jun 28. PMID:15990223 doi:http://dx.doi.org/10.1016/j.canlet.2005.04.024
- ↑ Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
- ↑ Cleophas MC, Joosten LA, Stamp LK, Dalbeth N, Woodward OM, Merriman TR. ABCG2 polymorphisms in gout: insights into disease susceptibility and treatment approaches. Pharmgenomics Pers Med. 2017 Apr 20;10:129-142. doi: 10.2147/PGPM.S105854., eCollection 2017. PMID:28461764 doi:http://dx.doi.org/10.2147/PGPM.S105854
- ↑ [ https://en.wikipedia.org/wiki/ABCG2 "ABCG2 -." Wikipedia, the Free Encyclopedia. Web. 20 Apr. 2020].
- ↑ Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
Student Contributors
Shelby Skaggs, Samuel Sullivan, Jaelyn Voyles