We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1613
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
One interesting feature of the NBD's is the fact that they remain in contact with one another even without a bound substrate. This makes the ABCG2 transporter unique and provides greater substrate specificity as the entrance to the transporter is not as globular as either ABCB1 or ABCC1. The entrance from the cytoplasm to the transporter is a [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] membrane entrance lined by <scene name='83/832939/Lining_of_entrance_of_nbd/1'>residues A397, V401, L405, L539, I543 and T547</scene> in both [https://en.wikipedia.org/wiki/Monomer monomers]. | One interesting feature of the NBD's is the fact that they remain in contact with one another even without a bound substrate. This makes the ABCG2 transporter unique and provides greater substrate specificity as the entrance to the transporter is not as globular as either ABCB1 or ABCC1. The entrance from the cytoplasm to the transporter is a [https://en.wikipedia.org/wiki/Hydrophobe hydrophobic] membrane entrance lined by <scene name='83/832939/Lining_of_entrance_of_nbd/1'>residues A397, V401, L405, L539, I543 and T547</scene> in both [https://en.wikipedia.org/wiki/Monomer monomers]. | ||
Dimerization of ABCG2 was originally thought to be achieved with the help of the <scene name='83/832939/Disproved_dimerization_process/1'>406xxx410 structural motif</scene> in each of the two domains but Cryo-EM showed that the [https://en.wikipedia.org/wiki/Sequence_motif motifs] were on opposite sides of the protein. | Dimerization of ABCG2 was originally thought to be achieved with the help of the <scene name='83/832939/Disproved_dimerization_process/1'>406xxx410 structural motif</scene> in each of the two domains but Cryo-EM showed that the [https://en.wikipedia.org/wiki/Sequence_motif motifs] were on opposite sides of the protein. | ||
| - | <ref name="Jackson">PMID:29610494</ref> | ||
<ref name="Manolaridis">PMID:30405239</ref> | <ref name="Manolaridis">PMID:30405239</ref> | ||
| + | <ref name="Jackson">PMID:29610494</ref> | ||
| + | |||
===Transmembrane Domain Stabilization=== | ===Transmembrane Domain Stabilization=== | ||
| Line 34: | Line 35: | ||
== Disease == | == Disease == | ||
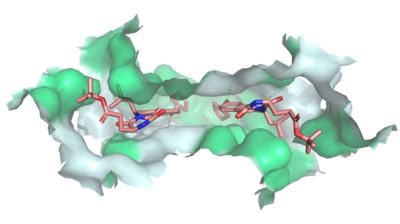
| - | One of the causes for multidrug resistant cancers is the excretion of cancer drugs out of the cell, thereby decreasing the effective intracellular concentration. ABCG2, also known as the breast cancer resistance protein (BCRP), effluxes multiple chemotherapeutic agents such as [https://en.wikipedia.org/wiki/Mitoxantrone mitoxantrone] and [https://en.wikipedia.org/wiki/Camptothecin camptothecin] analogies, making the cancerous breast cells resistant to chemotherapy. Competitive inhibitors, such as <scene name='83/832939/Abcg2_bound_to_mz29/3'>MZ29</scene>, shut down ABCG2 to stop the efflux of cancer drugs in order to combat the resistivity of breast cancer. <ref name="Jackson" / | + | One of the causes for multidrug resistant cancers is the excretion of cancer drugs out of the cell, thereby decreasing the effective intracellular concentration. ABCG2, also known as the breast cancer resistance protein (BCRP), effluxes multiple chemotherapeutic agents such as [https://en.wikipedia.org/wiki/Mitoxantrone mitoxantrone] and [https://en.wikipedia.org/wiki/Camptothecin camptothecin] analogies, making the cancerous breast cells resistant to chemotherapy. Competitive inhibitors, such as <scene name='83/832939/Abcg2_bound_to_mz29/3'>MZ29</scene>, shut down ABCG2 to stop the efflux of cancer drugs in order to combat the resistivity of breast cancer. <ref name="Jackson" /> |
The ABC transporter family has been found as a prevalent piece of multi-drug resistant cancers and therefore became a popular target towards inhibition. Three generations of drugs were made in order to inhibit a similar protein from the same family, ABCC1 at its interior binding site including cyclosporine A (first generation), valspodar (second generation), and Elacridar (3rd generation). Importantly, cyclosporine A and Elacridar were found to inhibit both ABCC1 and ABCG2 and in one trial had success along with chemotherapy in the treatment of acute myeloid leukemia but because of either side effects or experimentation that was not able to be duplicated, this research was mostly shelved. The main issue in their failure to find a drug to inhibit this protein was the failure to develop a high-resolution structure of this protein with the technology available at the time of this drug development. Now, with high resolution images of the structures, fourth generation inhibitors are being developed with more success in inhibition without additional drug interactions or cell toxicity. | The ABC transporter family has been found as a prevalent piece of multi-drug resistant cancers and therefore became a popular target towards inhibition. Three generations of drugs were made in order to inhibit a similar protein from the same family, ABCC1 at its interior binding site including cyclosporine A (first generation), valspodar (second generation), and Elacridar (3rd generation). Importantly, cyclosporine A and Elacridar were found to inhibit both ABCC1 and ABCG2 and in one trial had success along with chemotherapy in the treatment of acute myeloid leukemia but because of either side effects or experimentation that was not able to be duplicated, this research was mostly shelved. The main issue in their failure to find a drug to inhibit this protein was the failure to develop a high-resolution structure of this protein with the technology available at the time of this drug development. Now, with high resolution images of the structures, fourth generation inhibitors are being developed with more success in inhibition without additional drug interactions or cell toxicity. | ||
Revision as of 13:14, 21 April 2020
ABCG2 Transporter Protein
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018 Jul;18(7):452-464. doi: 10.1038/s41568-018-0005-8. PMID:29643473 doi:http://dx.doi.org/10.1038/s41568-018-0005-8
- ↑ 2.0 2.1 Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ 3.0 3.1 Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
- ↑ Fetsch PA, Abati A, Litman T, Morisaki K, Honjo Y, Mittal K, Bates SE. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006 Apr 8;235(1):84-92. doi: 10.1016/j.canlet.2005.04.024. Epub, 2005 Jun 28. PMID:15990223 doi:http://dx.doi.org/10.1016/j.canlet.2005.04.024
- ↑ Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
- ↑ Cleophas MC, Joosten LA, Stamp LK, Dalbeth N, Woodward OM, Merriman TR. ABCG2 polymorphisms in gout: insights into disease susceptibility and treatment approaches. Pharmgenomics Pers Med. 2017 Apr 20;10:129-142. doi: 10.2147/PGPM.S105854., eCollection 2017. PMID:28461764 doi:http://dx.doi.org/10.2147/PGPM.S105854
Student Contributors
Shelby Skaggs, Samuel Sullivan, Jaelyn Voyles