User:Neal Hayhurst/RNA Polymerase II/Sandbox 1
From Proteopedia
| Line 2: | Line 2: | ||
| - | == Introduction == | + | ==Introduction== |
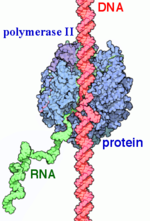
[[ Image:Label RNA pol II (1).png|150px|right|thumb| RNAP II transcription process.]] | [[ Image:Label RNA pol II (1).png|150px|right|thumb| RNAP II transcription process.]] | ||
| Line 22: | Line 22: | ||
Initially it was unknown if eukaryotes expressed one type of RNAP like eukaryotes or if there were multiple forms. In 1969 R. G. Roeder and and W. J. Rutter isolated three distinct species in sea urchin embryos by chromatography. They also showed that they required different environments for optimal activity and the forms are localized to different areas of the nucleus. RNAP I was found in the nucleous and RNAP II and III in the the nucleoplasm. Later experiments also showed that the different species if RNAP responded differently to to the inhibitor alpha-amanitin with RNAP being unresponsive to it, RNAP II inhibited by it, and RNAP II somewhere in between. Using this they could use varying concentrations of alpha-amanitin to see what types of RNA each RNAP was responsible for. <ref> DOI: 10.1038/nrm1796 </ref> | Initially it was unknown if eukaryotes expressed one type of RNAP like eukaryotes or if there were multiple forms. In 1969 R. G. Roeder and and W. J. Rutter isolated three distinct species in sea urchin embryos by chromatography. They also showed that they required different environments for optimal activity and the forms are localized to different areas of the nucleus. RNAP I was found in the nucleous and RNAP II and III in the the nucleoplasm. Later experiments also showed that the different species if RNAP responded differently to to the inhibitor alpha-amanitin with RNAP being unresponsive to it, RNAP II inhibited by it, and RNAP II somewhere in between. Using this they could use varying concentrations of alpha-amanitin to see what types of RNA each RNAP was responsible for. <ref> DOI: 10.1038/nrm1796 </ref> | ||
| - | == Structural Components == | ||
| - | == Structural Components == | + | |
| + | ==Structural Components== | ||
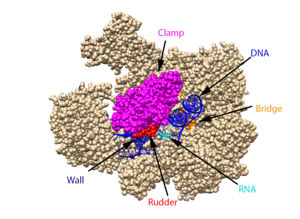
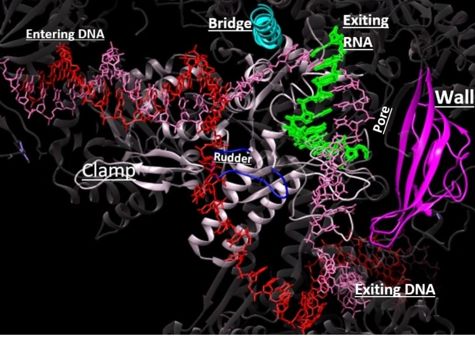
This section will briefly discuss the chief structural components involved in the mechanism. | This section will briefly discuss the chief structural components involved in the mechanism. | ||
| Line 95: | Line 95: | ||
| - | == α-Amanitin == | + | ==α-Amanitin== |
<scene name='86/862212/Alpha-amanitin/2'>α-Amanitin</scene> is a bicyclic octapeptide that adheres tightly with RNAP II and inhibits elongation during transcription. The <scene name='86/862212/Binding_site_of_alpha-amanitin/2'>binding site of α-amanitin</scene> is beneath a bridge helix that extends between a region of Rbp1 (funnel) and a region of Rbp2. The <scene name='86/862212/Hydroxyproline/2'>hydroxyproline of α-amanitin</scene> interacts with <scene name='86/862212/Bridge_helix_glu_822/2'>residue Glu 822 of the bridge helix</scene> via a hydrogen bond and the <scene name='86/862212/Ilx_gln_768_his_816/2'>4,5-dihydroxyisoleucine of α-amanitin</scene> interacts with residue Gln 768, which also interacts with residue His 816 of the bridge helix via a hydrogen bond. There are also hydrogen bonding interactions between some residues on the funnel region of Rbp1 and α-amanitin. The interaction between α-amanitin and the bridge helix restricts the movement of the bridge helix but it does not inhibit the entry of NTP into <scene name='86/862212/Alpha-amanitin_active_site/1'>RNA pol II’s active site</scene> (Brueckner et al, 2008) (Bushnell et al, 2001). The movement of the bridge helix is required for the translocation process, and the binding of α-amanitin with the bridge helix likely prevents the bridge’s conformational change that is necessary for the purposed RNAP translocation step (Brueckner et al, 2008) (Bushnell et al, 2001). Inhibition of the translocation of DNA and RNA results in the active site not being emptied for the next round of synthesis (Bushnell et al, 2001). α-Amanitin, found in the poisonous mushroom death cap, leads to death after several days. This time frame aligns with the rate at which mRNAs and proteins turnover. | <scene name='86/862212/Alpha-amanitin/2'>α-Amanitin</scene> is a bicyclic octapeptide that adheres tightly with RNAP II and inhibits elongation during transcription. The <scene name='86/862212/Binding_site_of_alpha-amanitin/2'>binding site of α-amanitin</scene> is beneath a bridge helix that extends between a region of Rbp1 (funnel) and a region of Rbp2. The <scene name='86/862212/Hydroxyproline/2'>hydroxyproline of α-amanitin</scene> interacts with <scene name='86/862212/Bridge_helix_glu_822/2'>residue Glu 822 of the bridge helix</scene> via a hydrogen bond and the <scene name='86/862212/Ilx_gln_768_his_816/2'>4,5-dihydroxyisoleucine of α-amanitin</scene> interacts with residue Gln 768, which also interacts with residue His 816 of the bridge helix via a hydrogen bond. There are also hydrogen bonding interactions between some residues on the funnel region of Rbp1 and α-amanitin. The interaction between α-amanitin and the bridge helix restricts the movement of the bridge helix but it does not inhibit the entry of NTP into <scene name='86/862212/Alpha-amanitin_active_site/1'>RNA pol II’s active site</scene> (Brueckner et al, 2008) (Bushnell et al, 2001). The movement of the bridge helix is required for the translocation process, and the binding of α-amanitin with the bridge helix likely prevents the bridge’s conformational change that is necessary for the purposed RNAP translocation step (Brueckner et al, 2008) (Bushnell et al, 2001). Inhibition of the translocation of DNA and RNA results in the active site not being emptied for the next round of synthesis (Bushnell et al, 2001). α-Amanitin, found in the poisonous mushroom death cap, leads to death after several days. This time frame aligns with the rate at which mRNAs and proteins turnover. | ||
Revision as of 03:15, 1 October 2020
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Young RA. RNA polymerase II. Annu Rev Biochem. 1991;60:689-715. doi: 10.1146/annurev.bi.60.070191.003353. PMID:1883205 doi:http://dx.doi.org/10.1146/annurev.bi.60.070191.003353
- ↑ 2.0 2.1 Myer VE, Young RA. RNA polymerase II holoenzymes and subcomplexes. J Biol Chem. 1998 Oct 23;273(43):27757-60. doi: 10.1074/jbc.273.43.27757. PMID:9774381 doi:http://dx.doi.org/10.1074/jbc.273.43.27757
- ↑ 3.0 3.1 3.2 Sobennikova MV, Shematorova EK, Shpakovskii GV. [C-terminal domain (CTD) of the subunit Rpb1 of nuclear RNA polymerase II and its role in the transcription cycle]. Mol Biol (Mosk). 2007 May-Jun;41(3):433-49. PMID:17685222
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 RNA polymerase II transcription initiation: A structural view D. B. Nikolov, S. K. Burley Proceedings of the National Academy of Sciences Jan 1997, 94 (1) 15-22; DOI: 10.1073/pnas.94.1.15
- ↑ Hurwitz J. The discovery of RNA polymerase. J Biol Chem. 2005 Dec 30;280(52):42477-85. doi: 10.1074/jbc.X500006200. Epub 2005, Oct 17. PMID:16230341 doi:http://dx.doi.org/10.1074/jbc.X500006200
- ↑ doi: https://dx.doi.org/10.1038/nrm1796
- ↑ 7.0 7.1 7.2 7.3 Orphanides, George, Thierry Lagrange, and Danny Reinberg. The general transcription factors of RNA polymerase II. Genes & development 10.21. 1996. 2657-2683
- ↑ 8.0 8.1 Xin, L.; Bushnell, D. A.; and Kornburg, R. D. RNA Polymerase II Transcription: Structure and Mechanism. Biochemica et Biophysica Acta. 2013, 1829, 2-8.
- ↑ Chang-Hui Shen; Diagnostic Molecular Biology, 2019
- ↑ He, Yuan, et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533.7603. 2016.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 Hahn, S. Structure and Mechanism of the RNA Polymerase II Transcription Machinery. Nature Structure and Molecular Biology. 2004, 11, 394-403.
- ↑ 12.0 12.1 Eick, D, Geyer, M.The RNA Polymerase II Carboxy-Terminal Domain (CTD) Code. Chemical Reviews, 2013, 113 (11), 8456-8490 DOI: 10.1021/cr400071f
- ↑ Wang W, Carey M, Gralla JD. Polymerase II Promoter Activation: Closed Complex Formation and ATP-Driven Start Site Opening. Science. 1992;255:450–453.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 14.8 14.9 Voet, D., Voet, J. G., & Pratt, C. W. (2013). Transcription and RNA Processing. In Fundamentals of biochemistry: life at the molecular level (pp. 933–942). Wiley.
- ↑ Nudler, E. RNA Polymerase Active Center: The Molecular Engine of Transcription. Annu. Rev. Biochem. 2009, 78, 335-361.
- ↑ Svetlov, V., & Nudler, E. (2013). Basic mechanism of transcription by RNA polymerase II. Biochimica et biophysica acta, 1829(1), 20–28. https://doi.org/10.1016/j.bbagrm.2012.08.009
- ↑ Castano E, Gross P, Wang Z, Roeder RG, Oelgeschlager T. The C-terminal domain-phosphorylated IIO form of RNA polymerase II is associated with the transcription repressor NC2 (Dr1/DRAP1) and is required for transcription activation in human nuclear extracts. Proc Natl Acad Sci U S A. 2000 Jun 20;97(13):7184-9. doi: 10.1073/pnas.140202297. PMID:10852970 doi:http://dx.doi.org/10.1073/pnas.140202297
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 18.7 18.8 Wang D, Bushnell DA, Westover KD, Kaplan CD, Kornberg RD. Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell. 2006 Dec 1;127(5):941-54. PMID:17129781 doi:http://dx.doi.org/10.1016/j.cell.2006.11.023
- ↑ 19.0 19.1 19.2 19.3 19.4 Mishanina TV, Palo MZ, Nayak D, Mooney RA, Landick R. Trigger loop of RNA polymerase is a positional, not acid-base, catalyst for both transcription and proofreading. Proc Natl Acad Sci U S A. 2017 Jun 27;114(26):E5103-E5112. doi:, 10.1073/pnas.1702383114. Epub 2017 Jun 12. PMID:28607053 doi:http://dx.doi.org/10.1073/pnas.1702383114
- ↑ 20.0 20.1 Da LT, Wang D, Huang X. Dynamics of pyrophosphate ion release and its coupled trigger loop motion from closed to open state in RNA polymerase II. J Am Chem Soc. 2012 Feb 1;134(4):2399-406. doi: 10.1021/ja210656k. Epub 2012 Jan , 24. PMID:22206270 doi:http://dx.doi.org/10.1021/ja210656k
- ↑ Bar-Nahum G, Epshtein V, Ruckenstein AE, Rafikov R, Mustaev A, Nudler E. A ratchet mechanism of transcription elongation and its control. Cell. 2005 Jan 28;120(2):183-93. doi: 10.1016/j.cell.2004.11.045. PMID:15680325 doi:http://dx.doi.org/10.1016/j.cell.2004.11.045
Bushnell, D. A.; Westover, K. D.; Davis, R. E.; Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II-TFIIB Cocrystal at 4.5 Angstroms. Science. 2004, 303, 983-988
Brueckner, F. and Cramer, P. Structural Basis of Transcription Inhibition by -amanitin and Implications for RNA Polymerase II Translocation. Nature Structure and Molecular Biology. 2008, 15, 811-818.
Cramer, P.; Bushnell, D. A.; Kornberg, R. D. Structural Basis of Transcription: RNA Polymerase II at 2.8 Ångstrom Resolution. Science. 2001, 292, 1863-1876
Evans, D. A.; Fitch, D. M.; Smith, T. E.; Cee, V. J. Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B. J. Am. Chem. Soc. 2000, 122, 10033-10046.
Gnatt, A. L.; Cramer, P; Fu, J.; Bushnell, D. A.; and Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II Elongation Complex at 3.3 Å Resolution. Science. 2001, 292, 1876-1882 1i6h
Hahn, S. Structure and Mechanism of the RNA Polymerase II Transcription Machinery. Nature Structure and Molecular Biology. 2004, 11, 394-403.
He, Yuan, et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533.7603. 2016.
Nudler, E. RNA Polymerase Active Center: The Molecular Engine of Transcription. Annu. Rev. Biochem. 2009, 78, 335-361.
Orphanides, George, Thierry Lagrange, and Danny Reinberg. The general transcription factors of RNA polymerase II. Genes & development 10.21. 1996. 2657-2683
Shah, N. et. al. Tyrosine-1 of RNA Polymerase II CTD Controls Global Termination of Gene Transcription in Mammals. Molecular Cell. 2018, 69, 48-61.
Uzman, A.; Voet, D. Student companion Fundamentals of biochemistry: life at the molecular level, 4th ed., Donald Voet, Judith G. Voet, Charlotte W. Pratt; John Wiley & amp; Sons, 2012.
Xu, J.; Lahiri, I.; Wang, W.; Wier, A.; Cianfrocco, M. A.; Chong, J.; Hare, A. A.; Dervan, P. B.; DiMaio, F.; Leschziner, A. E.; Wang, D. Structural Basis for the Initiation of Eukaryotic Transcription-coupled DNA Repair. Nature. 2017. 551, 653-657 5vvr
Xin, L.; Bushnell, D. A.; and Kornburg, R. D. RNA Polymerase II Transcription: Structure and Mechanism. Biochemica et Biophysica Acta. 2013, 1829, 2-8.
Yan, C., Dodd, T., He, Y., Tainer, J. A., Tsutakawa, S. E., & Ivanov, I. (2019). Transcription preinitiation complex structure and dynamics provide insight into genetic diseases. Nature Structural and Molecular Biology, 26(6), 397-406.
Eick, D, Geyer, M.The RNA Polymerase II Carboxy-Terminal Domain (CTD) Code. Chemical Reviews, 2013, 113 (11), 8456-8490 DOI: 10.1021/cr400071f
Chang-Hui Shen; Diagnostic Molecular Biology, 2019
Alpha-aminitin chemical structure image courtesy of https://en.wikipedia.org/wiki/Alpha-Amanitin#/media/File:Alpha-amanitin_structure.png
Notes
From structural components:
Structural overview: [PDB: 5VVR: with highlighted sections mentioned below]
Bridge: Depicted: [PDB: 1I6H: 810-845.a]
Wall: Depicted: [PDB: 1R5U: 853-919.b; 933-972.b]
Clamp: Depicted: [PDB: 1R5U: 3-345.a; 1395-1435.a; 1158-1124.b]
Rudder: Depicted: [PDB: 5VVR: 306-321.a]
Content Donators
This page was created as a final project for the Advanced Biochemistry course at Wabash College during the Fall of 2019 and Fall of 2020. This page was reviewed by Dr. Wally Novak of Wabash College.