Cavity programs
From Proteopedia
(→CASTp) |
(→CASTp) |
||
| Line 7: | Line 7: | ||
==CASTp== | ==CASTp== | ||
<table align="right" width="550" cellpadding="5" hspace="8" style="border: 1px solid black; border-collapse:collapse;padding-left:8px;"><tr><td> | <table align="right" width="550" cellpadding="5" hspace="8" style="border: 1px solid black; border-collapse:collapse;padding-left:8px;"><tr><td> | ||
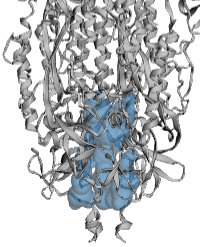
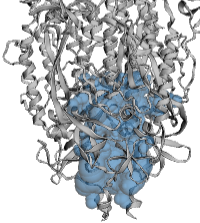
| - | <span style="font-size:120%; font-weight:bold;">CASTp:</span><br>Membrane-proximal cavity in the [[SARS-CoV-2 spike protein priming by furin|SARS-CoV-2 spike protein]] ([[6zgi]]) shown as a translucent envelope in blue | + | <span style="font-size:120%; font-weight:bold;">CASTp:</span><br>Membrane-proximal cavity in the [[SARS-CoV-2 spike protein priming by furin|SARS-CoV-2 spike protein]] ([[6zgi]]) shown as a translucent envelope in blue. |
| + | * Left: probe radius 2.5 Å. | ||
| + | * Right: CASTp default probe radius, 1.4 Å. | ||
</td><td> | </td><td> | ||
[[Image:Castp-6zgi-2.5.png|200px]] | [[Image:Castp-6zgi-2.5.png|200px]] | ||
Revision as of 20:01, 16 December 2020
This page lists programs that identify and offer visualization options for cavities in macromolecules. Broadly, the term "cavities" includes pockets, tunnels and channels. A pocket is a depression in the surface with one entrance. A tunnel connects two or more locations, and may or may not have entrances from the surface[1][2]. Some cavities are buried with no entrances from the surface (example: 3drf). These buried cavities are sometimes called voids[3].
Nearly all proteins have irregular surfaces with shallow pockets, mostly with no known functions. Some proteins have deep pockets, for example the catalytic anionic gorge in acetylcholinesterase (e. g. 1vot). Such a pocket can also be termed a tunnel accessing the catalytic site[1][2].
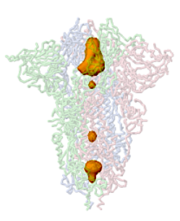
The example below is the membrane-proximal cavity of SARS-CoV-2 spike protein (6zgi). The membrane-proximal cavity is a potential target for drugs to prevent membrane fusion, and thus prevent infection. Programs are listed alphabetically.
Contents |
CASTp
|
CASTp:
|
CASTp: Computed Atlas of Surface Topography of proteins. "CASTp is based on recent theorectical and algorithmic results of Computational Geometry. It has many advantages: 1) pockets and cavities are identified analytically, 2) the boundary between the bulk solvent and the pocket is defined precisely, 3) all calculated parameters are rotationally invariant, and do not involve discretization and they make no use of dot surface or grid points." (See Comparison Note[4])
Displays protein sequences indicating which residues line the displayed cavities. Clicking on a residue centers the 3D view accordingly.
Web server visualization is in 3Dmol.js, which seems to offer no user-customizable options beyond rotate and zoom, such as centering or hiding the protein cartoon.
CASTp 3.0 published summer, 2018[5]. Available as a web server, and a PyMOL plugin. Results can be downloaded for offline viewing with the PyMOL plugin.
Jmol
|
Jmol: |
Jmol can identify and display pockets and cavities as isosurfaces. Examples are shown at Jmol/Cavities pockets and tunnels, where you will also find explanations of the interior cavity and pocket commands.
Jmol has an extensive command language that provides great flexibility in visualizing cavities.
The Jmol Java standalone application is downloadable from jmol.org. It is also available as JSmol, a Javascript implementation used in most pages in Proteopedia.
Jmol is updated many times each year.
PACUPP
|
PACUPP:
|
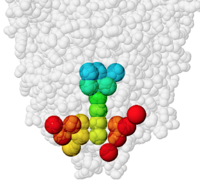
PACUPP, Pockets And Cavities Using Pseudoatoms in Proteins, identifies cavities by filling them with pseudoatoms (holmium, Ho, think "holes"). An example is presented in some detail at PACUPP: Pockets And Cavities Using Pseudoatoms in Proteins. Further examples with demonstrations of how to use PACUPP are in a YouTube video and a slideshow, available from molviz.org/pacupp, where you can also download the program.
PACUPP offers a number of simple commands specialized for visualizing cavities, mostly single letter commands. Some call up a dialog where the use enters information. Lists cavity-lining atoms in a spreadsheet-ready text file. Learning the PACUPP commands is much easier than learning Jmol commands.
PACUPP is a Jmol script. It processes 2/3 of the entries in the Protein Data Bank in ≤15 sec each. For large models such as ribosomes or proteasomes that may take many minutes, PACUPP offers an unattended batch mode.
First released December, 2020.
References
- ↑ 1.0 1.1 Marques SM, Daniel L, Buryska T, Prokop Z, Brezovsky J, Damborsky J. Enzyme Tunnels and Gates As Relevant Targets in Drug Design. Med Res Rev. 2017 Sep;37(5):1095-1139. doi: 10.1002/med.21430. Epub 2016 Dec 13. PMID:27957758 doi:http://dx.doi.org/10.1002/med.21430
- ↑ 2.0 2.1 Kingsley LJ, Lill MA. Substrate tunnels in enzymes: structure-function relationships and computational methodology. Proteins. 2015 Apr;83(4):599-611. doi: 10.1002/prot.24772. Epub 2015 Feb 28. PMID:25663659 doi:http://dx.doi.org/10.1002/prot.24772
- ↑ The CASTp server uses the term void.
- ↑ In contrast with CASTP, PACUPP uses grid points. Therefore, its cavity boundaries are slightly different when the grid points are offset by half of the spacing between points -- an option it offers. See Offset: Hit or Miss & Cavity Volume in How To Use PACUPP.
- ↑ Tian W, Chen C, Lei X, Zhao J, Liang J. CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res. 2018 Jul 2;46(W1):W363-W367. doi: 10.1093/nar/gky473. PMID:29860391 doi:http://dx.doi.org/10.1093/nar/gky473