We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Abbey Wells/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structure == | == Structure == | ||
=== Overall Structure === | === Overall Structure === | ||
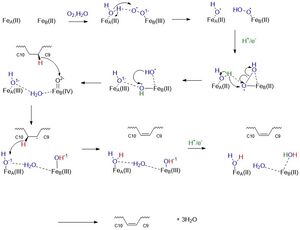
| - | SCD1 is an integral membrane protein embedded within the [http://micro.magnet.fsu.edu/cells/endoplasmicreticulum/endoplasmicreticulum.html endoplasmic reticulum] and consists of 4 transmembrane [http://en.wikipedia.org/wiki/Alpha_helix alpha helices], arranged in a cone-like shape. The cytosolic domain of the enzyme consists of 11 alpha helices and contains the carboxy and amino termini <ref name="Bai">PMID:26098370</ref>. Its substrate, Stearoyl-CoA, binds to the cytosolic region which contains a "kink" that properly orients Stearoyl-CoA to undergo a [http://en.wikipedia.org/wiki/Dehydrogenation#:~:text=Dehydrogenation%20is%20the%20a%20chemical,reaction%20and%20a%20serious%20problem.&text=Enzymes%20that%20catalyze%20dehydrogenation%20are%20called%20dehydrogenases. dehydrogenation] reaction between the <scene name='87/877602/C9_and_c10/ | + | SCD1 is an integral membrane protein embedded within the [http://micro.magnet.fsu.edu/cells/endoplasmicreticulum/endoplasmicreticulum.html endoplasmic reticulum] and consists of 4 transmembrane [http://en.wikipedia.org/wiki/Alpha_helix alpha helices], arranged in a cone-like shape. The cytosolic domain of the enzyme consists of 11 alpha helices and contains the carboxy and amino termini <ref name="Bai">PMID:26098370</ref>. Its substrate, Stearoyl-CoA, binds to the cytosolic region which contains a "kink" that properly orients Stearoyl-CoA to undergo a [http://en.wikipedia.org/wiki/Dehydrogenation#:~:text=Dehydrogenation%20is%20the%20a%20chemical,reaction%20and%20a%20serious%20problem.&text=Enzymes%20that%20catalyze%20dehydrogenation%20are%20called%20dehydrogenases. dehydrogenation] reaction between the <scene name='87/877602/C9_and_c10/2'>carbon 9 and carbon 10</scene> of Stearoyl-CoA. |
=== Binding of Substrate === | === Binding of Substrate === | ||
Revision as of 12:55, 20 April 2021
Stearoyl-CoA Desaturase 1 from Mus musculus
| |||||||||||
References
- ↑ 1.0 1.1 Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009 Jul;297(1):E28-37. doi:, 10.1152/ajpendo.90897.2008. Epub 2008 Dec 9. PMID:19066317 doi:http://dx.doi.org/10.1152/ajpendo.90897.2008
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Bai Y, McCoy JG, Levin EJ, Sobrado P, Rajashankar KR, Fox BG, Zhou M. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature. 2015 Jun 22. doi: 10.1038/nature14549. PMID:26098370 doi:http://dx.doi.org/10.1038/nature14549
- ↑ 3.0 3.1 Castro LF, Wilson JM, Goncalves O, Galante-Oliveira S, Rocha E, Cunha I. The evolutionary history of the stearoyl-CoA desaturase gene family in vertebrates. BMC Evol Biol. 2011 May 19;11:132. doi: 10.1186/1471-2148-11-132. PMID:21595943 doi:http://dx.doi.org/10.1186/1471-2148-11-132
- ↑ Shen J, Wu G, Tsai AL, Zhou M. Structure and Mechanism of a Unique Diiron Center in Mammalian Stearoyl-CoA Desaturase. J Mol Biol. 2020 May 27. pii: S0022-2836(20)30367-3. doi:, 10.1016/j.jmb.2020.05.017. PMID:32470559 doi:http://dx.doi.org/10.1016/j.jmb.2020.05.017
Student Contributors
- Abbey Wells
- Josey McKinley
- Anthony Durand