We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Abbey Wells/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
[http://en.wikipedia.org/wiki/Stearoyl-CoA Stearoyl-CoA] is the substrate that binds to the enzyme, SCD1. The binding of the substrate is stabilized by specific residues on the exterior and interior of the protein. Stearoyl-CoA is a long-chain fatty [http://en.wikipedia.org/wiki/Acyl-CoA#:~:text=Acyl%2DCoA%20is%20a%20group,forming%20several%20equivalents%20of%20ATP. acyl-CoA]. The head group of the substrate is composed of an adenine, ribose, phosphate groups, and [http://en.wikipedia.org/wiki/Chemical_polarity#:~:text=In%20chemistry%2C%20polarity%20is%20a,electronegativity%20between%20the%20bonded%20atoms polar atoms] such as of nitrogen, oxygen, and sulfur. The head of stearoyl-CoA is attached to the exterior of the protein by polar residues. The adenine, ribose, and phosphate are attached by the residues <scene name='87/877602/Hydrophillic_top_t/2'>R151, D152, K185</scene>. The remaining exterior of the substrate is attached by the residues <scene name='87/877602/Hydrophillic_bottom_labeled_t/2'>N144, N71, R184</scene>. All the conserved residues are attached to the Stearoyl-CoA via [http://en.wikipedia.org/wiki/Hydrogen_bond#:~:text=A%20hydrogen%20bond%20(often%20informally,donor%20(Dn)%E2%80%94and%20another hydrogen bonds]. The fatty acid tail of Stearoyl-CoA is a 17-carbon chain which reaches into the interior of the protein. The fatty acid chain dives into the interior hydrophobic tunnel which is long, narrow, and approximately 24 Angstroms long <ref name="Bai">PMID:26098370</ref>. The geometry of the tunnel and formation of bound acyl chain are the structural basis for the stereospecificity of the desaturation reaction <ref name="Bai">PMID:26098370</ref>. | [http://en.wikipedia.org/wiki/Stearoyl-CoA Stearoyl-CoA] is the substrate that binds to the enzyme, SCD1. The binding of the substrate is stabilized by specific residues on the exterior and interior of the protein. Stearoyl-CoA is a long-chain fatty [http://en.wikipedia.org/wiki/Acyl-CoA#:~:text=Acyl%2DCoA%20is%20a%20group,forming%20several%20equivalents%20of%20ATP. acyl-CoA]. The head group of the substrate is composed of an adenine, ribose, phosphate groups, and [http://en.wikipedia.org/wiki/Chemical_polarity#:~:text=In%20chemistry%2C%20polarity%20is%20a,electronegativity%20between%20the%20bonded%20atoms polar atoms] such as of nitrogen, oxygen, and sulfur. The head of stearoyl-CoA is attached to the exterior of the protein by polar residues. The adenine, ribose, and phosphate are attached by the residues <scene name='87/877602/Hydrophillic_top_t/2'>R151, D152, K185</scene>. The remaining exterior of the substrate is attached by the residues <scene name='87/877602/Hydrophillic_bottom_labeled_t/2'>N144, N71, R184</scene>. All the conserved residues are attached to the Stearoyl-CoA via [http://en.wikipedia.org/wiki/Hydrogen_bond#:~:text=A%20hydrogen%20bond%20(often%20informally,donor%20(Dn)%E2%80%94and%20another hydrogen bonds]. The fatty acid tail of Stearoyl-CoA is a 17-carbon chain which reaches into the interior of the protein. The fatty acid chain dives into the interior hydrophobic tunnel which is long, narrow, and approximately 24 Angstroms long <ref name="Bai">PMID:26098370</ref>. The geometry of the tunnel and formation of bound acyl chain are the structural basis for the stereospecificity of the desaturation reaction <ref name="Bai">PMID:26098370</ref>. | ||
=== Kink of Chain === | === Kink of Chain === | ||
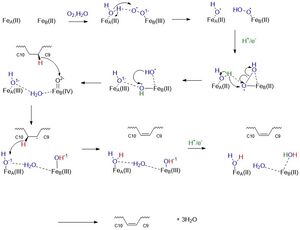
| - | The chain is kinked at <scene name='87/877602/C9_and_c10/2'>carbon 9 and carbon 10</scene> where the double bond is generated. The kink is induced through the interactions of four conserved residues. Three out of four of these residues are not bound to the chain, but are hydrogen bonded to each other: <scene name='87/877602/Kink_build/2'>T257, Q143, W149</scene>. T257 is hydrogen bonded to Q143, and Q143 is hydrogen bonded to W149 <ref name="Bai">PMID:26098370</ref>. These residues are directly below the kink and will be hydrolyzed when the enzymatic product is ready to be released. Specifically, if the hydrogen bond between T257 and Q143 is broken, a large opening would allow for the product to be released into the bilayer <ref name="Bai">PMID:26098370</ref>. The residue that is directly hydrogen bonded to the chain is <scene name='87/877602/W258/ | + | The chain is kinked at <scene name='87/877602/C9_and_c10/2'>carbon 9 and carbon 10</scene> where the double bond is generated. The kink is induced through the interactions of four conserved residues. Three out of four of these residues are not bound to the chain, but are hydrogen bonded to each other: <scene name='87/877602/Kink_build/2'>T257, Q143, W149</scene>. T257 is hydrogen bonded to Q143, and Q143 is hydrogen bonded to W149 <ref name="Bai">PMID:26098370</ref>. These residues are directly below the kink and will be hydrolyzed when the enzymatic product is ready to be released. Specifically, if the hydrogen bond between T257 and Q143 is broken, a large opening would allow for the product to be released into the bilayer <ref name="Bai">PMID:26098370</ref>. The residue that is directly hydrogen bonded to the chain is <scene name='87/877602/W258/2'>W258</scene>. This residue is highly conserved and stabilizes the chain so it will be in the correct orientation in the active site. The enzyme will be effective on acyl chains that are between 17 to 19 carbons long. The residue that has a role in determining substrate length is <scene name='87/877602/Cap/2'>Y104</scene>. Y104 is a capping residue that has approximately 4 [http://en.wikipedia.org/wiki/Angstrom Angstroms] between its' hydroxyl oxygen and the end of the chain <ref name="Bai">PMID:26098370</ref>. |
=== Active Site === | === Active Site === | ||
Revision as of 13:11, 20 April 2021
Stearoyl-CoA Desaturase 1 from Mus musculus
| |||||||||||
References
- ↑ 1.0 1.1 Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009 Jul;297(1):E28-37. doi:, 10.1152/ajpendo.90897.2008. Epub 2008 Dec 9. PMID:19066317 doi:http://dx.doi.org/10.1152/ajpendo.90897.2008
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Bai Y, McCoy JG, Levin EJ, Sobrado P, Rajashankar KR, Fox BG, Zhou M. X-ray structure of a mammalian stearoyl-CoA desaturase. Nature. 2015 Jun 22. doi: 10.1038/nature14549. PMID:26098370 doi:http://dx.doi.org/10.1038/nature14549
- ↑ 3.0 3.1 Castro LF, Wilson JM, Goncalves O, Galante-Oliveira S, Rocha E, Cunha I. The evolutionary history of the stearoyl-CoA desaturase gene family in vertebrates. BMC Evol Biol. 2011 May 19;11:132. doi: 10.1186/1471-2148-11-132. PMID:21595943 doi:http://dx.doi.org/10.1186/1471-2148-11-132
- ↑ Shen J, Wu G, Tsai AL, Zhou M. Structure and Mechanism of a Unique Diiron Center in Mammalian Stearoyl-CoA Desaturase. J Mol Biol. 2020 May 27. pii: S0022-2836(20)30367-3. doi:, 10.1016/j.jmb.2020.05.017. PMID:32470559 doi:http://dx.doi.org/10.1016/j.jmb.2020.05.017
Student Contributors
- Abbey Wells
- Josey McKinley
- Anthony Durand