This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox GGC15
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='1A36' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1A36' size='340' side='right' caption='Caption for this structure' scene=''> | ||
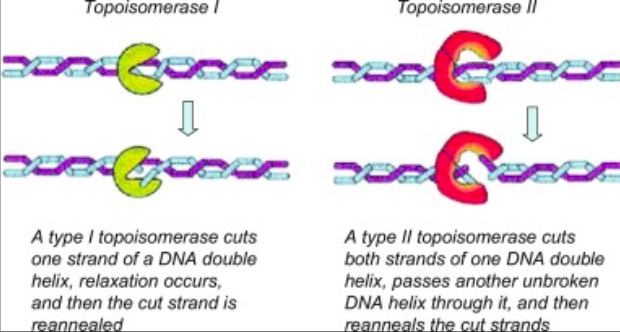
Eukaryotic DNA topoisomerase I (topo I) is a protein that reduces the strain from the supercoils that are caused during transcription and translation<ref name="Staker">DOI 10.1073/pnas.242259599</ref>. There are two types of topoisomerases. Type 1 topoisomerases are monomeric and break one strand of DNA<ref name="Redinbo">PMID:9488644</ref>. Type 2 topoisomerases are dimeric, meaning that they made up of two units and break both strands of the DNA helix<ref name="Redinbo" />. They are able to pass another part of the duplex through the cut, and close the cut using ATP<ref name="Staker" />. | Eukaryotic DNA topoisomerase I (topo I) is a protein that reduces the strain from the supercoils that are caused during transcription and translation<ref name="Staker">DOI 10.1073/pnas.242259599</ref>. There are two types of topoisomerases. Type 1 topoisomerases are monomeric and break one strand of DNA<ref name="Redinbo">PMID:9488644</ref>. Type 2 topoisomerases are dimeric, meaning that they made up of two units and break both strands of the DNA helix<ref name="Redinbo" />. They are able to pass another part of the duplex through the cut, and close the cut using ATP<ref name="Staker" />. | ||
| - | + | [[Image:04_27_21_A136_Top_1_and_Top_2_Example.jpg]]. <ref name="Dyakonov">D'yakonov, V. A., Dzhemileva, L. U., & Dzhemilev, U. M. (2017). Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Studies in Natural Products Chemistry, 21–86. https://doi.org/10.1016/b978-0-444-63929-5.00002-4 </ref>. | |
== Structure == | == Structure == | ||
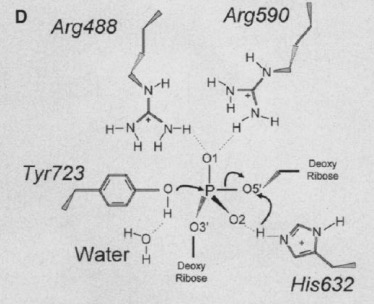
Human topo 1 is composed of 765 amino acids <ref name="Redinbo" />. The enzyme consist of 4 regions which are the NH2-terminal, core, linker, and COOH-terminal domains<ref name="Redinbo" />. The NH2-terminal is approximately 210 residues long, it is highly charged, disordered, and contains few hydrophobic amino acids<ref name="Redinbo" />. The COOH-terminal domain is made up of residues 713 to 765 and contains the important amino aside Tyrosine 223<ref name="Redinbo"/>. The location of the active site is at this amino acid<ref name="Redinbo" />. | Human topo 1 is composed of 765 amino acids <ref name="Redinbo" />. The enzyme consist of 4 regions which are the NH2-terminal, core, linker, and COOH-terminal domains<ref name="Redinbo" />. The NH2-terminal is approximately 210 residues long, it is highly charged, disordered, and contains few hydrophobic amino acids<ref name="Redinbo" />. The COOH-terminal domain is made up of residues 713 to 765 and contains the important amino aside Tyrosine 223<ref name="Redinbo"/>. The location of the active site is at this amino acid<ref name="Redinbo" />. | ||
Revision as of 22:13, 27 April 2021
DNA TOPOISOMERASE I
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15387-92. Epub 2002 Nov 8. PMID:12426403 doi:10.1073/pnas.242259599
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998 Mar 6;279(5356):1504-13. PMID:9488644
- ↑ D'yakonov, V. A., Dzhemileva, L. U., & Dzhemilev, U. M. (2017). Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Studies in Natural Products Chemistry, 21–86. https://doi.org/10.1016/b978-0-444-63929-5.00002-4
- ↑ Stewart, L. (1998). A Model for the Mechanism of Human Topoisomerase I. Science, 279(5356), 1534–1541. https://doi.org/10.1126/science.279.5356.1534