We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox GGC15
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
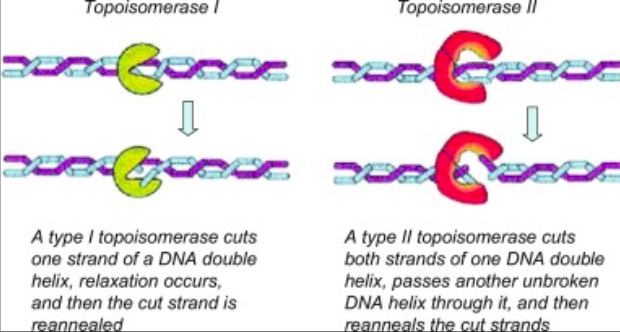
Eukaryotic DNA topoisomerase I (topo I) is a protein that reduces the strain from the supercoils that are caused during transcription and translation<ref name="Staker">DOI 10.1073/pnas.242259599</ref>. There are two types of topoisomerases. Type 1 topoisomerases are monomeric and break one strand of DNA<ref name="Redinbo">PMID:9488644</ref>. Type 2 topoisomerases are dimeric, meaning that they made up of two units and break both strands of the DNA helix<ref name="Redinbo" />. They are able to pass another part of the duplex through the cut, and close the cut using ATP<ref name="Staker" />. | Eukaryotic DNA topoisomerase I (topo I) is a protein that reduces the strain from the supercoils that are caused during transcription and translation<ref name="Staker">DOI 10.1073/pnas.242259599</ref>. There are two types of topoisomerases. Type 1 topoisomerases are monomeric and break one strand of DNA<ref name="Redinbo">PMID:9488644</ref>. Type 2 topoisomerases are dimeric, meaning that they made up of two units and break both strands of the DNA helix<ref name="Redinbo" />. They are able to pass another part of the duplex through the cut, and close the cut using ATP<ref name="Staker" />. | ||
[[Image:04_27_21_A136_Top_1_and_Top_2_Example.jpg]]. <ref name="Dyakonov">D'yakonov, V. A., Dzhemileva, L. U., & Dzhemilev, U. M. (2017). Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Studies in Natural Products Chemistry, 21–86. https://doi.org/10.1016/b978-0-444-63929-5.00002-4 </ref>. | [[Image:04_27_21_A136_Top_1_and_Top_2_Example.jpg]]. <ref name="Dyakonov">D'yakonov, V. A., Dzhemileva, L. U., & Dzhemilev, U. M. (2017). Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Studies in Natural Products Chemistry, 21–86. https://doi.org/10.1016/b978-0-444-63929-5.00002-4 </ref>. | ||
| + | |||
== Structure == | == Structure == | ||
| - | Human topo 1 is composed of 765 amino acids <ref name="Redinbo" />. The enzyme consist of 4 regions which are the NH2-terminal, core, linker, and COOH-terminal domains<ref name="Redinbo" />. The NH2-terminal is approximately 210 residues long, it is highly charged, disordered, and contains few hydrophobic amino acids<ref name="Redinbo" />. The COOH-terminal domain is made up of residues 713 to 765 and contains the important amino aside Tyrosine 223<ref name="Redinbo"/>. The location of the active site is at this amino acid<ref name="Redinbo" />. | + | Human topo 1 is composed of 765 amino acids <ref name="Redinbo" />. The enzyme consist of 4 regions which are the NH2-terminal, core, linker, and COOH-terminal domains<ref name="Redinbo" />. The NH2-terminal is approximately 210 residues long, it is highly charged, disordered, and contains few hydrophobic amino acids<ref name="Redinbo" />. The COOH-terminal domain is made up of residues 713 to 765 and contains the important amino aside Tyrosine 223<ref name="Redinbo"/>. The location of the active site is at this amino acid<ref name="Redinbo" />. Residues 636 to 712 form the linker domain and they contribute to the enzyme catalytic activity but are not required<ref name="Redinbo" />. The core and the COOH-terminal domain are very important for the catalytic activity<ref name="Redinbo" />. |
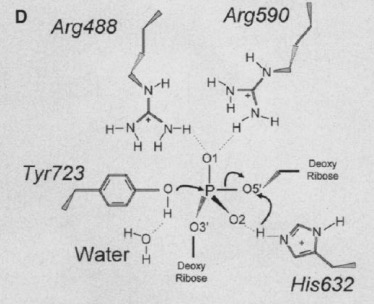
== Active Site == | == Active Site == | ||
Revision as of 23:38, 27 April 2021
DNA TOPOISOMERASE I
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15387-92. Epub 2002 Nov 8. PMID:12426403 doi:10.1073/pnas.242259599
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998 Mar 6;279(5356):1504-13. PMID:9488644
- ↑ D'yakonov, V. A., Dzhemileva, L. U., & Dzhemilev, U. M. (2017). Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Studies in Natural Products Chemistry, 21–86. https://doi.org/10.1016/b978-0-444-63929-5.00002-4
- ↑ Stewart, L. (1998). A Model for the Mechanism of Human Topoisomerase I. Science, 279(5356), 1534–1541. https://doi.org/10.1126/science.279.5356.1534
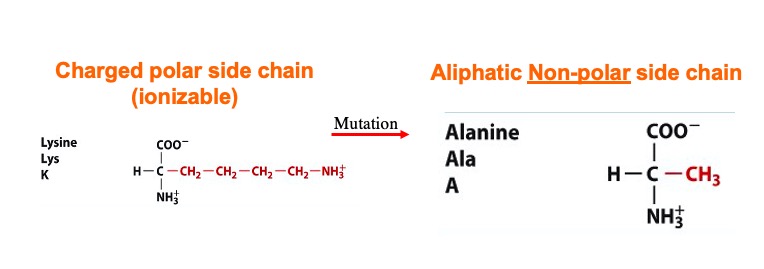
- ↑ 5.0 5.1 Interthal H, Quigley PM, Hol WG, Champoux JJ. The role of lysine 532 in the catalytic mechanism of human topoisomerase I. J Biol Chem. 2004 Jan 23;279(4):2984-92. Epub 2003 Oct 31. PMID:14594810 doi:10.1074/jbc.M309959200