Sandbox GGC15
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Structure == | == Structure == | ||
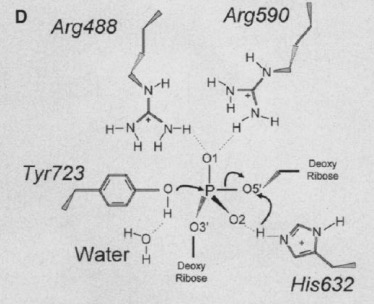
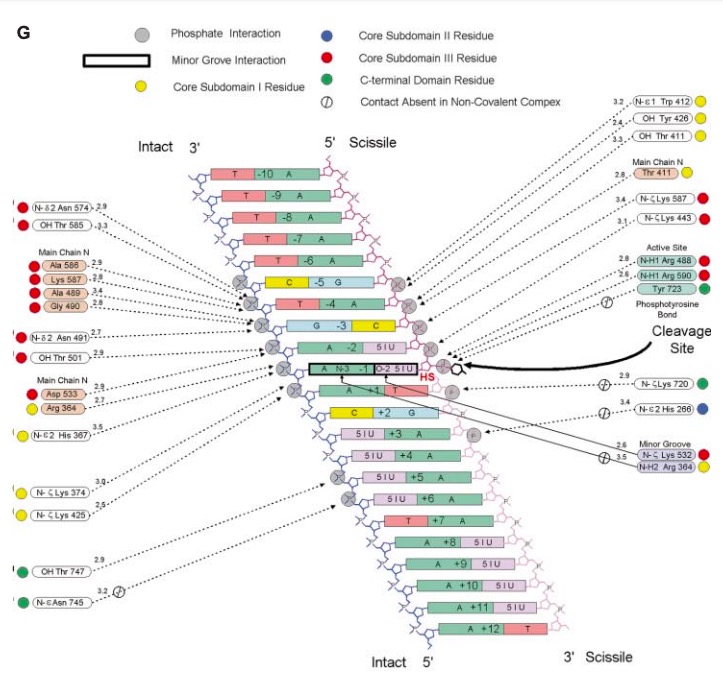
| - | '''Human topo 1 is composed of 765 amino acids <ref name="Redinbo" />. The enzyme consist of 4 regions which are the NH2-terminal, core, linker, and COOH-terminal domains<ref name="Redinbo" />. The NH2-terminal is approximately 210 residues long, it is highly charged, disordered, and contains few hydrophobic amino acids<ref name="Redinbo" />. The <scene name='78/781215/04_27_structure_cooh/1'>COOH-terminal</scene> domain is made up of residues 713 to 765 and contains the important amino aside Tyrosine 723<ref name="Redinbo"/>. The location of the active site is at this amino acid<ref name="Redinbo" />. Residues 636 to 712 form the <scene name='78/781215/04_27_structure_linker/1'>Linker domain</scene> and they contribute to the enzyme catalytic activity but are not required<ref name="Redinbo" />. The core is divided into 3 subdomains: <scene name='78/781215/04_27_structure_core_sd1/1'>Core Sudomain 1</scene>[215-232 & 320-433], <scene name='78/781215/04_27_structure_core_sd2/1'>Core Subdomain 2</scene>[233-319], and <scene name='78/781215/04_27_structure_core_sd3/1'>Core Domain 3</scene>[434-635]<ref name="Redinbo"/>. The core and the COOH-terminal domain are very important for the catalytic activity<ref name="Redinbo" />.''' | + | '''Human topo 1 is composed of 765 amino acids <ref name="Redinbo" />. The enzyme consist of 4 regions which are the NH2-terminal, core, linker, and COOH-terminal domains<ref name="Redinbo" />. The NH2-terminal is approximately 210 residues long, it is highly charged, disordered, and contains few hydrophobic amino acids<ref name="Redinbo" />. The <scene name='78/781215/04_27_structure_cooh/1'>COOH-terminal</scene> domain is made up of residues 713 to 765 and contains the important amino aside <scene name='78/781215/04_27_structure_cooh_with_k/1'>Tyrosine 723</scene><ref name="Redinbo"/>. The location of the active site is at this amino acid<ref name="Redinbo" />. Residues 636 to 712 form the <scene name='78/781215/04_27_structure_linker/1'>Linker domain</scene> and they contribute to the enzyme catalytic activity but are not required<ref name="Redinbo" />. The core is divided into 3 subdomains: <scene name='78/781215/04_27_structure_core_sd1/1'>Core Sudomain 1</scene>[215-232 & 320-433], <scene name='78/781215/04_27_structure_core_sd2/1'>Core Subdomain 2</scene>[233-319], and <scene name='78/781215/04_27_structure_core_sd3/1'>Core Domain 3</scene>[434-635]<ref name="Redinbo"/>. The core and the COOH-terminal domain are very important for the catalytic activity<ref name="Redinbo" />.''' |
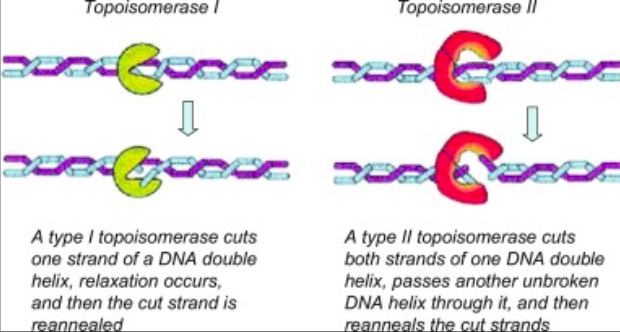
== Active Site == | == Active Site == | ||
Revision as of 01:36, 28 April 2021
DNA TOPOISOMERASE I
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15387-92. Epub 2002 Nov 8. PMID:12426403 doi:10.1073/pnas.242259599
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WG. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science. 1998 Mar 6;279(5356):1504-13. PMID:9488644
- ↑ D'yakonov, V. A., Dzhemileva, L. U., & Dzhemilev, U. M. (2017). Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Studies in Natural Products Chemistry, 21–86. https://doi.org/10.1016/b978-0-444-63929-5.00002-4
- ↑ 4.0 4.1 Stewart, L. (1998). A Model for the Mechanism of Human Topoisomerase I. Science, 279(5356), 1534–1541. https://doi.org/10.1126/science.279.5356.1534
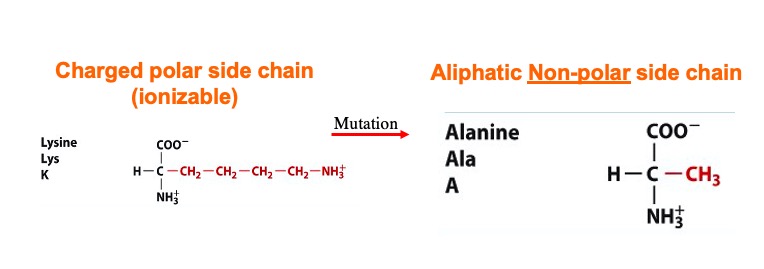
- ↑ 5.0 5.1 Interthal H, Quigley PM, Hol WG, Champoux JJ. The role of lysine 532 in the catalytic mechanism of human topoisomerase I. J Biol Chem. 2004 Jan 23;279(4):2984-92. Epub 2003 Oct 31. PMID:14594810 doi:10.1074/jbc.M309959200