This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Image:Obraz 1.jpg
From Proteopedia
Size of this preview: 736 × 600 pixels
Full resolution (757 × 617 pixel, file size: 230 KB, MIME type: image/jpeg)
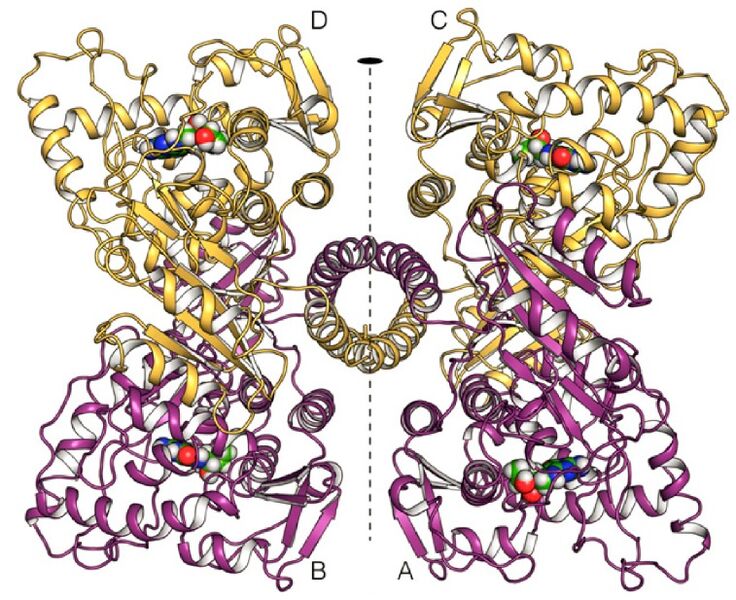
(Fig. 1. Crystal structure of human tetrameric PAH. Each monomere binds BH4 molecule as a cofactor which is shown here as spheres. The homo-tetrameric PAH has a two-fold symetry, which is drawn here as dashed black line (Flydal, M. I. et al., 2019)) |
(Fig. 1. Crystal structure of human tetrameric PAH. Each monomere binds BH4 molecule as a cofactor which is shown here as spheres. The homo-tetrameric PAH has a two-fold symetry, which is drawn here as dashed black line (Flydal, M. I. et al., 2019)) |
Current revision
Fig. 1. Crystal structure of human tetrameric PAH. Each monomere binds BH4 molecule as a cofactor which is shown here as spheres. The homo-tetrameric PAH has a two-fold symetry, which is drawn here as dashed black line (Flydal, M. I. et al., 2019)
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 19:32, 30 April 2021 | Alexandra Gredová (Talk | contribs) | 757×617 | 230 KB | Fig. 1. Crystal structure of human tetrameric PAH. Each monomere binds BH4 molecule as a cofactor which is shown here as spheres. The homo-tetrameric PAH has a two-fold symetry, which is drawn here as dashed black line (Flydal, M. I. et al., 2019) |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
