We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1694
From Proteopedia
(Difference between revisions)
| Line 32: | Line 32: | ||
In the structure INPP1D54A, there is a mutation in the amino acid aspartic acid (D)54 and causes it to change to alanine (A)54 (<scene name='89/892737/Alanine/1'>mutation in D54</scene>). This mutation does not impact the substrate affinity but does decrease the activity of INPP1. <ref name="dollins" /> | In the structure INPP1D54A, there is a mutation in the amino acid aspartic acid (D)54 and causes it to change to alanine (A)54 (<scene name='89/892737/Alanine/1'>mutation in D54</scene>). This mutation does not impact the substrate affinity but does decrease the activity of INPP1. <ref name="dollins" /> | ||
[[Image:Motif_Copy.png | thumb]] | [[Image:Motif_Copy.png | thumb]] | ||
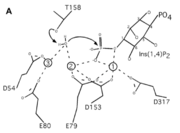
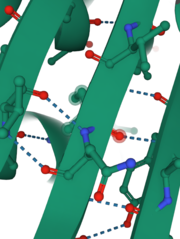
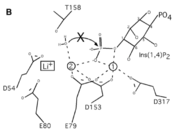
| - | A six amino acid <scene name='89/892737/Motif/1'>motif</scene>, DPIDxT anchors the metal-binding sites in the protein that are likely involved in catalysis while the metal binds to the substrate. <ref name="dollins" /> The sixth amino acid, x, is not as important as the other five, however, it can be any amino acid depending on the related crystallized structure to INPP1D54A or to a similar protein. Also, lithium is an uncompetitive inhibitor for this enzyme and when it binds to a metal site (metal site 3 in the lower image B) it causes the | + | A six amino acid <scene name='89/892737/Motif/1'>motif</scene>, DPIDxT anchors the metal-binding sites in the protein that are likely involved in catalysis while the metal binds to the substrate. <ref name="dollins" /> The sixth amino acid, x, is not as important as the other five, however, it can be any amino acid depending on the related crystallized structure to INPP1D54A or to a similar protein. Also, lithium is an uncompetitive inhibitor for this enzyme and when it binds to a metal site (metal site 3 in the lower image B) it causes the enzyme not to function properly. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references /> | <references /> | ||
Revision as of 15:08, 9 December 2021
| This Sandbox is Reserved from 10/01/2021 through 01/01//2022 for use in Biochemistry taught by Bonnie Hall at Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1690 through Sandbox Reserved 1699. |
To get started:
More help: Help:Editing |
Inositol polyphosphate 1-Phosphatase (INPP1) D54A
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 Dollins DE, Xiong JP, Endo-Streeter S, Anderson DE, Bansal VS, Ponder JW, Ren Y, York JD. A Structural Basis for Lithium and Substrate Binding of an Inositide Phosphatase. J Biol Chem. 2020 Nov 10. pii: RA120.014057. doi: 10.1074/jbc.RA120.014057. PMID:33172890 doi:http://dx.doi.org/10.1074/jbc.RA120.014057