User:Mathias Bortoletto Dunker/Sandbox 1
From Proteopedia
(Difference between revisions)
m |
|||
| Line 36: | Line 36: | ||
|} | |} | ||
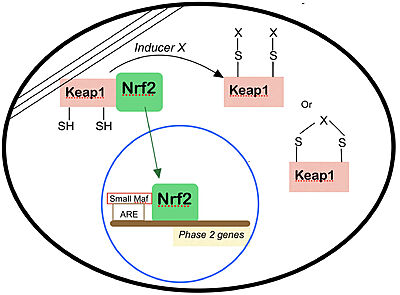
| - | == | + | == Mechanism and function of Keap 1 == |
In eukaryote cells, the defense against reactive molecules from both endogenous or exogenous sources are usually coordinated thanks to the expression of genes regulated by the Cap N' Collar transcription factors, a unique subset within the [https://en.wikipedia.org/wiki/BZIP_domain bZIP] family of transcription factors.<ref> Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;(71):157-76. doi: 10.1042/bss0710157. PMID: 15777020. </ref> Such molecules (reactive oxygen species, electrophilic chemicals and heavy metals), if not neutralized and eliminated, can affect the oxidation levels of most endocytic molecules and compromise the integrity of the DNA, leading to severe damage to the organism and causing many pathophysiological processes, including many known diseases in humans such as cancer<ref>Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001 Jun 2;477(1-2):7-21. doi: 10.1016/s0027-5107(01)00091-4. PMID: 11376682. </ref>and cardiovascular problems. <ref>Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003 Dec 15;420(2):217-21. doi: 10.1016/j.abb.2003.06.002. PMID: 14654060. </ref> The expression of genes regulated by the Cap N' Collar (CNC) set — which includes the transcription factors Nrf1, Nrf2, Nrf3, Bach1 and Bach2 — are indispensable for the induction of enzymes that can neutralize such reactive molecules, eliminate damaged macromolecules and restore cellular redox homeostasis.<ref name="main" /> | In eukaryote cells, the defense against reactive molecules from both endogenous or exogenous sources are usually coordinated thanks to the expression of genes regulated by the Cap N' Collar transcription factors, a unique subset within the [https://en.wikipedia.org/wiki/BZIP_domain bZIP] family of transcription factors.<ref> Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;(71):157-76. doi: 10.1042/bss0710157. PMID: 15777020. </ref> Such molecules (reactive oxygen species, electrophilic chemicals and heavy metals), if not neutralized and eliminated, can affect the oxidation levels of most endocytic molecules and compromise the integrity of the DNA, leading to severe damage to the organism and causing many pathophysiological processes, including many known diseases in humans such as cancer<ref>Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001 Jun 2;477(1-2):7-21. doi: 10.1016/s0027-5107(01)00091-4. PMID: 11376682. </ref>and cardiovascular problems. <ref>Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003 Dec 15;420(2):217-21. doi: 10.1016/j.abb.2003.06.002. PMID: 14654060. </ref> The expression of genes regulated by the Cap N' Collar (CNC) set — which includes the transcription factors Nrf1, Nrf2, Nrf3, Bach1 and Bach2 — are indispensable for the induction of enzymes that can neutralize such reactive molecules, eliminate damaged macromolecules and restore cellular redox homeostasis.<ref name="main" /> | ||
| Line 58: | Line 58: | ||
[[Image:Met.jpg|400px|center|]] | [[Image:Met.jpg|400px|center|]] | ||
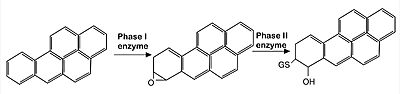
Image: representation of endocytic metabolisation of reactive agents (in this case, Benoz[a]pyrine molecules). The xenobiotic agent is first oxidized by phase I enzymes belonging to the P450 mono-oxygenase system (such as CYP1A1 and CYP1A2 enzymes). Since the product of such reactions are often highly reactive, they are subsequently detoxified by the induction of enzymes related to Phase II reactions (such as glutathione S-transferase and UDP–glucuronosyl-transferase), that promote the conjugation of phase I products with hydrophilic moieties, such as glutathione and glucuronic acid, so that the final, non-reactive product can be then eliminated by excretion. Nrf2 is the main CNC transcription factor responsible for the induction of those Phase II enzymes. | Image: representation of endocytic metabolisation of reactive agents (in this case, Benoz[a]pyrine molecules). The xenobiotic agent is first oxidized by phase I enzymes belonging to the P450 mono-oxygenase system (such as CYP1A1 and CYP1A2 enzymes). Since the product of such reactions are often highly reactive, they are subsequently detoxified by the induction of enzymes related to Phase II reactions (such as glutathione S-transferase and UDP–glucuronosyl-transferase), that promote the conjugation of phase I products with hydrophilic moieties, such as glutathione and glucuronic acid, so that the final, non-reactive product can be then eliminated by excretion. Nrf2 is the main CNC transcription factor responsible for the induction of those Phase II enzymes. | ||
| - | == Structural and Genetic | + | == Structural and Genetic Information of Nrf2 == |
NRF2 is 605 amino acids long and possesses <scene name='89/898348/Nrf2inteirafinal/1'>seven highly conserved domains</scene> called NRF2-ECH homology (Neh) domains <ref>Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034</ref>. Their names and function is as follows: | NRF2 is 605 amino acids long and possesses <scene name='89/898348/Nrf2inteirafinal/1'>seven highly conserved domains</scene> called NRF2-ECH homology (Neh) domains <ref>Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034</ref>. Their names and function is as follows: | ||
| Line 76: | Line 76: | ||
[[Image:Nrf2gene.jpg|500px|center|]] | [[Image:Nrf2gene.jpg|500px|center|]] | ||
| - | == | + | == Structural and Genetic Information of Keap1 == |
== Structural highlights == | == Structural highlights == | ||
Revision as of 04:52, 13 December 2021
Keap1-Nrf2 Complex
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243
- ↑ Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020
- ↑ Data available in https://www.ncbi.nlm.nih.gov/gene/9817
- ↑ Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;(71):157-76. doi: 10.1042/bss0710157. PMID: 15777020.
- ↑ Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001 Jun 2;477(1-2):7-21. doi: 10.1016/s0027-5107(01)00091-4. PMID: 11376682.
- ↑ Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003 Dec 15;420(2):217-21. doi: 10.1016/j.abb.2003.06.002. PMID: 14654060.
- ↑ Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003 Nov 28;278(48):48021-9. doi: 10.1074/jbc.M308439200. Epub 2003 Sep 10. PMID: 12968018.
- ↑ 8.0 8.1 Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11908-13. doi: 10.1073/pnas.172398899. Epub 2002 Aug 22. PMID: 12193649; PMCID: PMC129367.
- ↑ 9.0 9.1 9.2 Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004 Nov;10(11):549-57. doi: 10.1016/j.molmed.2004.09.003. PMID: 15519281.
- ↑ Sasaki, H. et al. (2002) Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 277, 44765–44771
- ↑ Chan, K. and Kan, Y.W. (1999) Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. U. S. A. 96, 12731–12736
- ↑ Enomoto, A. et al. (2001) High sensitivity of Nrf2 knockout mice to Review TRENDS in Molecular Medicine Vol.10 No.11 November 2004 555 www.sciencedirect.com acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 59, 169–177
- ↑ Goldring, C.E. et al. (2004) Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 39, 1267–1276
- ↑ Ramos-Gomez, M. et al. (2001) Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 98, 3410–3415
- ↑ Cho, H-Y. et al. (2002) Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 26, 175–182
- ↑ Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034