User:Mathias Bortoletto Dunker/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='2Flu' size='340' side='right' caption='Crystal Structure of the Kelch-Neh2 Complex' scene='89/898348/Flu2/1'> | <StructureSection load='2Flu' size='340' side='right' caption='Crystal Structure of the Kelch-Neh2 Complex' scene='89/898348/Flu2/1'> | ||
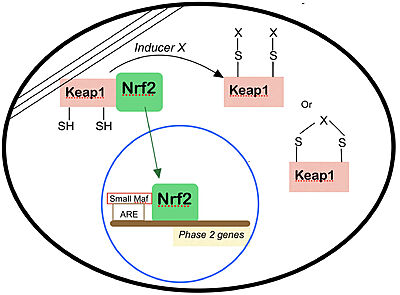
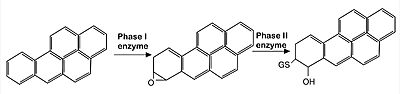
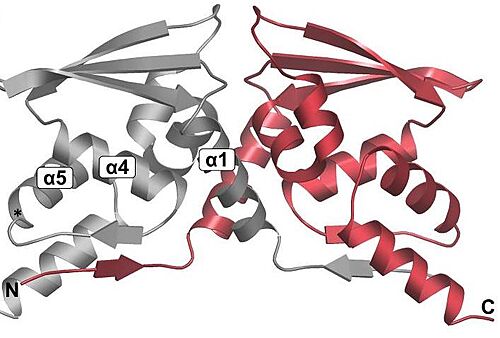
| - | Keap1 is a BTB-Kelch substrate adaptor protein anchored to the actin cytoskeleton that regulates steady-state levels of [https://en.wikipedia.org/wiki/NFE2L2 Nrf2],<ref name="main">Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243 </ref> one of the main transcription factor of cytoprotective genes found in mammals.<ref>Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020</ref> Under homeostatic conditions, Keap1 is capable of marking Nfr2 for [https://en.wikipedia.org/wiki/Ubiquitin ubiquitin-dependent degradation], repressing the expression of genes associated with cytological defense against highly reactive molecules, such as electrophilic chemicals, heavy metals and oxidative agents. <ref name="main" /> As such, when the cell is subjected to stresses of those kinds, Nrf2 is no longer targeted for ubiquitin-dependent degradation, thus allowing for the expressions of the genes responsible for the cellular defense against such reactive substances. | + | Keap1 is a BTB-Kelch substrate adaptor protein anchored to the actin cytoskeleton that regulates steady-state levels of [https://en.wikipedia.org/wiki/NFE2L2 Nrf2],<ref name="main">Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243 </ref> one of the main transcription factor of cytoprotective genes found in mammals.<ref>Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020</ref> Under homeostatic conditions, <scene name='89/898348/Flu2/1'>Keap1 is capable of marking Nfr2 </scene> for [https://en.wikipedia.org/wiki/Ubiquitin ubiquitin-dependent degradation], repressing the expression of genes associated with cytological defense against highly reactive molecules, such as electrophilic chemicals, heavy metals and oxidative agents. <ref name="main" /> As such, when the cell is subjected to stresses of those kinds, Nrf2 is no longer targeted for ubiquitin-dependent degradation, thus allowing for the expressions of the genes responsible for the cellular defense against such reactive substances. |
{| class="wikitable" | {| class="wikitable" | ||
Revision as of 13:32, 13 December 2021
Keap1-Nrf2 Complex
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Lo, S.-C., Li, X., Henzl, M.T., Beamer, L.J. and Hannink, M. (2006), Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO Journal, 25: 3605-3617. https://doi.org/10.1038/sj.emboj.7601243
- ↑ Pitoniak, A., & Bohmann, D. (2015). Mechanisms and functions of Nrf2 signaling in Drosophila. Free radical biology & medicine, 88(Pt B), 302–313. https://doi.org/10.1016/j.freeradbiomed.2015.06.020
- ↑ Data available in https://www.ncbi.nlm.nih.gov/gene/9817
- ↑ Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;(71):157-76. doi: 10.1042/bss0710157. PMID: 15777020.
- ↑ Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001 Jun 2;477(1-2):7-21. doi: 10.1016/s0027-5107(01)00091-4. PMID: 11376682.
- ↑ Ceconi C, Boraso A, Cargnoni A, Ferrari R. Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys. 2003 Dec 15;420(2):217-21. doi: 10.1016/j.abb.2003.06.002. PMID: 14654060.
- ↑ Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003 Nov 28;278(48):48021-9. doi: 10.1074/jbc.M308439200. Epub 2003 Sep 10. PMID: 12968018.
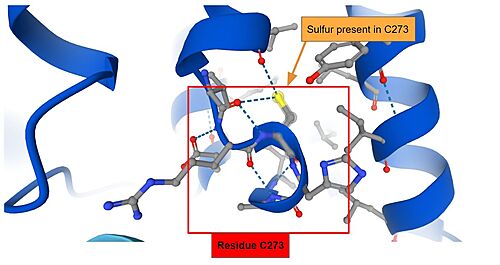
- ↑ 8.0 8.1 Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11908-13. doi: 10.1073/pnas.172398899. Epub 2002 Aug 22. PMID: 12193649; PMCID: PMC129367.
- ↑ 9.0 9.1 9.2 Albena T. Dinkova-Kostova, W. David Holtzclaw, and Thomas W. Kensler. The Role of Keap1 in Cellular Protective Responses. American Chemical Society, August 2, 2005.
- ↑ Nobunao Wakabayashi, Albena T. Dinkova-Kostova, W. David Holtzclaw, Moon-Il Kang, Akira Kobayashi, Masayuki Yamamoto, Thomas W. Kensler, and Paul Talalay. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. PNAS February 17, 2004 vol. 101 no. 7.
- ↑ 11.0 11.1 11.2 Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004 Nov;10(11):549-57. doi: 10.1016/j.molmed.2004.09.003. PMID: 15519281.
- ↑ Sasaki, H. et al. (2002) Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J. Biol. Chem. 277, 44765–44771
- ↑ Chan, K. and Kan, Y.W. (1999) Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. U. S. A. 96, 12731–12736
- ↑ Enomoto, A. et al. (2001) High sensitivity of Nrf2 knockout mice to Review TRENDS in Molecular Medicine Vol.10 No.11 November 2004 555 www.sciencedirect.com acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 59, 169–177
- ↑ Goldring, C.E. et al. (2004) Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 39, 1267–1276
- ↑ Ramos-Gomez, M. et al. (2001) Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 98, 3410–3415
- ↑ Cho, H-Y. et al. (2002) Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 26, 175–182
- ↑ Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034
- ↑ https://www.annualreviews.org/doi/full/10.1146/annurev-cancerbio-030518-055627, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=94063579
- ↑ Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101-107. doi:10.1016/j.freeradbiomed.2015.05.034
- ↑ Cleasby A, Yon J, Day PJ, et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS One. 2014;9(6):e98896. Published 2014 Jun 4. doi:10.1371/journal.pone.0098896
- ↑ Woycechowsky, K. J., and Raines, R. T. (2003) The CXC motif: a functional mimic of protein disulfide isomerase. Biochemistry 42, 5387-5394.