Dihydrofolate reductase
From Proteopedia
| Line 3: | Line 3: | ||
__TOC__ | __TOC__ | ||
== Sources == | == Sources == | ||
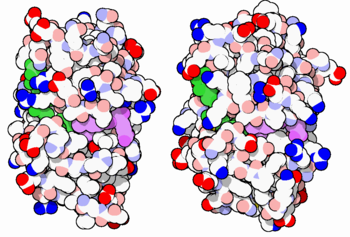
| - | DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli and humans have similar folds, while the plasmid-encoded enzyme has an unrelated fold. | ||
[[Image:34-DihydrofolateReductase-3dfr-1dls.PNG|thumb|350px|E. coli (left) and human (right) DHFR have a similar architecture and mode of binding to NADPH(green) and methotrexate(purple). Original image by David Goodsell]] | [[Image:34-DihydrofolateReductase-3dfr-1dls.PNG|thumb|350px|E. coli (left) and human (right) DHFR have a similar architecture and mode of binding to NADPH(green) and methotrexate(purple). Original image by David Goodsell]] | ||
| + | |||
| + | DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli and humans have similar folds, while the plasmid-encoded enzyme has an unrelated fold. | ||
== Reactions catalyzed == | == Reactions catalyzed == | ||
Revision as of 17:45, 6 January 2022
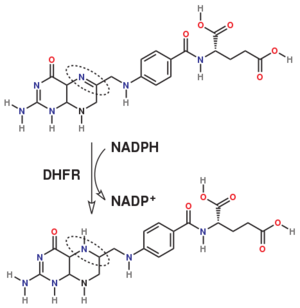
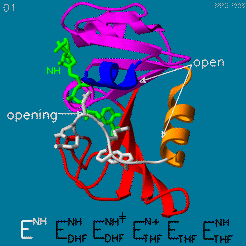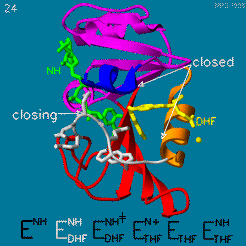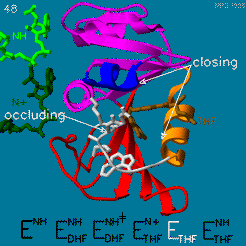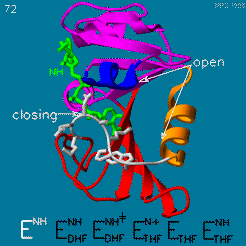
The enzyme dihydrofolate reductase (DHFR) occurs in all organisms and has been particularly well-studied in the bacterium Escherichia coli and in humans[1]. It catalyzes the reduction of dihydrofolate to tetrahydrofolate, with NADPH acting as hydride donor. The human enzyme is a target for developing anti-cancer chemotherapies, while the bacterial enzymes are targets for developing antibiotics. DHFR is a model enzyme for studying the kinetics, mechanism, and inhibition of enzymatic reactions and the underlying structure and conformational dynamics.
Contents |
Sources
DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli and humans have similar folds, while the plasmid-encoded enzyme has an unrelated fold.
Reactions catalyzed
Dihydrofolate reductase (DHFR) is an enzyme which uses the co-factor NADPH as electron donor which converts it to NADP. It catalyzes the reduction of dihydrofolic acid to tetrahydrofolic acid (folate).
The folate is a form of the essential vitamin B9.
Relevance
DHFR forms a complex with thymidylate synthase (TS)[2]. Both enzymes participate in the biosynthesis of pyrimidine.[3]
Your Heading Here (maybe something like 'Structure')
| |||||||||||
See also
Relevance
Antifolate inhibitors like Methotrexate (MTX) which is very similar to folic acid are used in cancer therapy.
3D Structures of Dihydrofolate reductase
Dihydrofolate reductase 3D structures
Additional Resources
- For additional information, see: Cancer.
- See also Molecular Playground/DHFR.
References
- ↑ Schnell JR, Dyson HJ, Wright PE. Structure, dynamics, and catalytic function of dihydrofolate reductase. Annu Rev Biophys Biomol Struct. 2004;33:119-40. doi:, 10.1146/annurev.biophys.33.110502.133613. PMID:15139807 doi:http://dx.doi.org/10.1146/annurev.biophys.33.110502.133613
- ↑ Ivanetich KM, Santi DV. Thymidylate synthase-dihydrofolate reductase in protozoa. Exp Parasitol. 1990 Apr;70(3):367-71. PMID:2178951
- ↑ Bolin JT, Filman DJ, Matthews DA, Hamlin RC, Kraut J. Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 A resolution. I. General features and binding of methotrexate. J Biol Chem. 1982 Nov 25;257(22):13650-62. PMID:6815178
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Alexander Berchansky, Joel L. Sussman, Tzvia Selzer, Jaime Prilusky, Eric Martz, Eran Hodis, David Canner