Dihydrofolate reductase
From Proteopedia
| Line 2: | Line 2: | ||
| - | == | + | == DHFR from different organisms == |
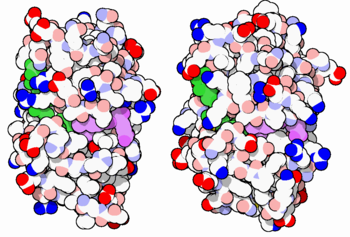
[[Image:34-DihydrofolateReductase-3dfr-1dls.PNG|thumb|350px|E. coli (left) and human (right) DHFR have a similar architecture and mode of binding to NADPH(green) and the competitive inhibitor methotrexate(purple). Original image by David Goodsell]] | [[Image:34-DihydrofolateReductase-3dfr-1dls.PNG|thumb|350px|E. coli (left) and human (right) DHFR have a similar architecture and mode of binding to NADPH(green) and the competitive inhibitor methotrexate(purple). Original image by David Goodsell]] | ||
| - | DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli and humans have similar folds, while the plasmid-encoded enzyme has an unrelated fold. In humans, DHFR is expressed in most tissues[https://www.proteinatlas.org/ENSG00000228716-DHFR], and there are two genes, DHFR and DHFR2/DHFRL1, the latter targeted to mitochondria<ref>DOI:10.1073/pnas.1103605108</ref>. Mice and rats lack the second gene but also show DHFR activity in mitochondria<ref>DOI:10.1016/j.febslet.2015.05.017</ref>. | + | DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli (ecDHFR) and humans (hDHFR) have similar folds, while the plasmid-encoded enzyme has an unrelated fold. In humans, DHFR is expressed in most tissues[https://www.proteinatlas.org/ENSG00000228716-DHFR], and there are two genes, DHFR and DHFR2/DHFRL1, the latter targeted to mitochondria<ref>DOI:10.1073/pnas.1103605108</ref>. Mice and rats lack the second gene but also show DHFR activity in mitochondria<ref>DOI:10.1016/j.febslet.2015.05.017</ref>. |
== Reactions catalyzed == | == Reactions catalyzed == | ||
| Line 42: | Line 42: | ||
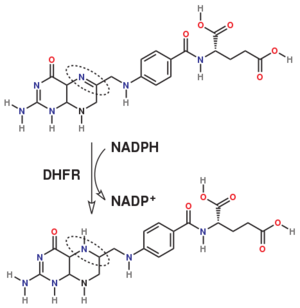
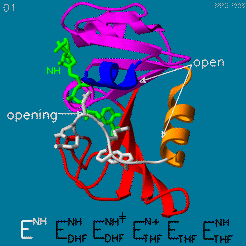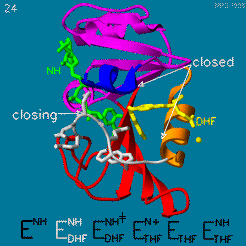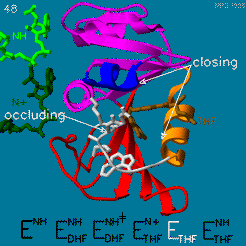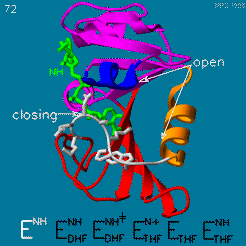
DHFR is thought to proceed in a multi-step mechanism. Once NADPH and DHF are bound to the E. coli enzyme, DHF is first protonated and then reduced through a hydride transfer from NADPH.<ref>doi:10.1073/pnas.1415940111</ref> Substrate binding and product release is thought to have a definite choreography, with fresh NADPH binding before THF is released. | DHFR is thought to proceed in a multi-step mechanism. Once NADPH and DHF are bound to the E. coli enzyme, DHF is first protonated and then reduced through a hydride transfer from NADPH.<ref>doi:10.1073/pnas.1415940111</ref> Substrate binding and product release is thought to have a definite choreography, with fresh NADPH binding before THF is released. | ||
| - | The protonation step was studied using neutron diffraction and high-resolution X-ray diffractionto visualize the hydrogen atoms that are usually not visible in crystal structures.<ref>doi:10.1073/pnas.1415856111</ref> | + | The <scene name='82/82636/Active_site/1'>protonation step</scene> was studied using neutron diffraction and high-resolution X-ray diffractionto visualize the hydrogen atoms that are usually not visible in crystal structures.<ref>doi:10.1073/pnas.1415856111</ref> |
The hydride transfer is thought to involve hydride tunneling, supported by temperature-dependent kinetic isotope effects. Tunneling is a quantum phenomenon explaining how a small particle can cross an activation barrier even when it lacks sufficient activation energy. <ref>doi:10.3390/quantum3010006</ref> | The hydride transfer is thought to involve hydride tunneling, supported by temperature-dependent kinetic isotope effects. Tunneling is a quantum phenomenon explaining how a small particle can cross an activation barrier even when it lacks sufficient activation energy. <ref>doi:10.3390/quantum3010006</ref> | ||
Revision as of 02:51, 7 January 2022
The enzyme dihydrofolate reductase (DHFR) occurs in all organisms and has been particularly well-studied in the bacterium Escherichia coli and in humans[1][2][3]. It catalyzes the reduction of dihydrofolate to tetrahydrofolate, with NADPH acting as hydride donor. The human enzyme is a target for developing inhibitors used in anti-cancer chemotherapies, while the bacterial enzymes are targets for developing inhibitors as antibiotics. DHFR is a model enzyme for studying the kinetics, mechanism, and inhibition of enzymatic reactions and the underlying structure and conformational dynamics.
Contents |
DHFR from different organisms
DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli (ecDHFR) and humans (hDHFR) have similar folds, while the plasmid-encoded enzyme has an unrelated fold. In humans, DHFR is expressed in most tissues[1], and there are two genes, DHFR and DHFR2/DHFRL1, the latter targeted to mitochondria[4]. Mice and rats lack the second gene but also show DHFR activity in mitochondria[5].
Reactions catalyzed
Dihydrofolate reductase (DHFR, 1.5.1.3 [2]) is an enzyme which uses the co-factor NADPH as electron donor which converts it to NADP. It catalyzes the reduction of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF). The mammalian enzymes also accept folic acid as a substrate, reducing it to THF. This allows the use of folic acid, which is easier to synthesize than DHF or THF, to fortify food.[6]. Some bacterial enzymes also accept folic acid as a substrate [7] but it acts as a competitive inhibitor in the E. coli enzyme.
The folate is a form of the essential vitamin B9. Folate is not part of our natural diet (it contains dihydrofolate and tetrahydrofolate, sometimes as a poly-glutamate conjugate) but is bioavailable and simpler to synthesize.
Relevance
DHFR forms a complex with thymidylate synthase (TS)[8]. Both enzymes participate in the biosynthesis of pyrimidine.[9]
| |||||||||||
See also
3D Structures of Dihydrofolate reductase
Dihydrofolate reductase 3D structures
Additional Resources
- For additional information, see: Cancer.
- See also Molecular Playground/DHFR.
References
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Alexander Berchansky, Joel L. Sussman, Tzvia Selzer, Jaime Prilusky, Eric Martz, Eran Hodis, David Canner