Dihydrofolate reductase
From Proteopedia
| Line 46: | Line 46: | ||
The hydride transfer is thought to involve hydride tunneling, supported by temperature-dependent kinetic isotope effects. Tunneling is a quantum phenomenon explaining how a small particle can cross an activation barrier even when it lacks sufficient activation energy. <ref>doi:10.3390/quantum3010006</ref> | The hydride transfer is thought to involve hydride tunneling, supported by temperature-dependent kinetic isotope effects. Tunneling is a quantum phenomenon explaining how a small particle can cross an activation barrier even when it lacks sufficient activation energy. <ref>doi:10.3390/quantum3010006</ref> | ||
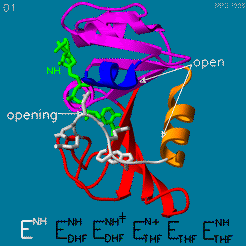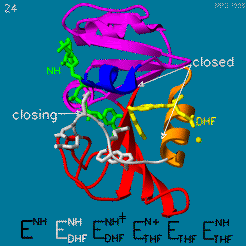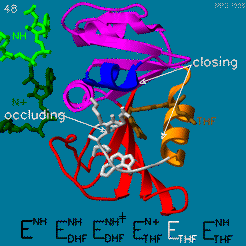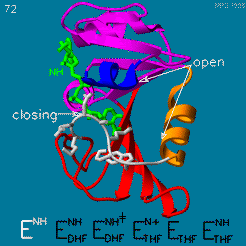
| - | The domain orientation and the Met20 loop conformations changes during the catalytic cycle. A model by M. R. Sawaya and J. Kraut from 1997 summarizing these motions based on six isomorphous crystal structures is shown in the movie below. The original web page of this movie is available on the [https://web.archive.org/web/19991003061611/http://chem-faculty.ucsd.edu/kraut/dhfr.html web archive]. | + | The domain orientation and the Met20 loop conformations changes during the catalytic cycle. A model by M. R. Sawaya and J. Kraut from 1997 summarizing these motions based on six isomorphous crystal structures is shown in the movie below <ref>doi:10.1021/bi962337c</ref>. The original web page of this movie is available on the [https://web.archive.org/web/19991003061611/http://chem-faculty.ucsd.edu/kraut/dhfr.html web archive]. |
Revision as of 03:21, 7 January 2022
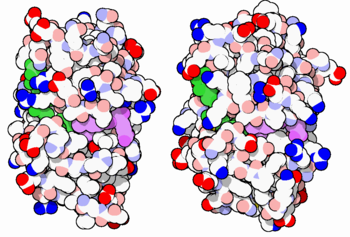
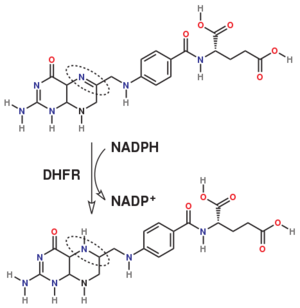
The enzyme dihydrofolate reductase (DHFR) occurs in all organisms and has been particularly well-studied in the bacterium Escherichia coli and in humans[1][2][3]. It catalyzes the reduction of dihydrofolate to tetrahydrofolate, with NADPH acting as hydride donor. The human enzyme is a target for developing inhibitors used in anti-cancer chemotherapies[4], while the bacterial enzymes are targets for developing inhibitors as antibiotics. DHFR is a model enzyme for studying the kinetics, mechanism, and inhibition of enzymatic reactions and the underlying structure and conformational dynamics.
Contents |
DHFR from different organisms
DHFR is found in all organisms. Some bacteria acquire resistance to DHFR inhibitors through expressing a second form of DHFR coded on a plasmid. The enzymes from E. coli (ecDHFR) and humans (hDHFR) have similar folds, while the plasmid-encoded enzyme has an unrelated fold. In humans, DHFR is expressed in most tissues[1], and there are two genes, DHFR and DHFR2/DHFRL1, the latter targeted to mitochondria[5]. Mice and rats lack the second gene but also show DHFR activity in mitochondria[6].
Reactions catalyzed
Dihydrofolate reductase (DHFR, 1.5.1.3 [2]) is an enzyme which uses the co-factor NADPH as electron donor which converts it to NADP. It catalyzes the reduction of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF). The mammalian enzymes also accept folic acid as a substrate, reducing it to THF. This allows the use of folic acid, which is easier to synthesize than DHF or THF, to fortify food.[7]. Some bacterial enzymes also accept folic acid as a substrate [8] but it acts as a competitive inhibitor in the E. coli enzyme.
The folate is a form of the essential vitamin B9. Folate is not part of our natural diet (it contains dihydrofolate and tetrahydrofolate, sometimes as a poly-glutamate conjugate) but is bioavailable and simpler to synthesize.
Relevance
DHFR forms a complex with thymidylate synthase (TS)[9]. Both enzymes participate in the biosynthesis of pyrimidine.[10]
| |||||||||||
See also
3D Structures of Dihydrofolate reductase
Dihydrofolate reductase 3D structures
Additional Resources
- For additional information, see: Cancer.
- See also Molecular Playground/DHFR.
References
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Alexander Berchansky, Joel L. Sussman, Tzvia Selzer, Jaime Prilusky, Eric Martz, Eran Hodis, David Canner