We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1653
From Proteopedia
(Difference between revisions)
| Line 107: | Line 107: | ||
Piezo1 is a protein discovered recently. Therefore, its potential in medicine is in constant evolution. | Piezo1 is a protein discovered recently. Therefore, its potential in medicine is in constant evolution. | ||
| - | Piezo1 can be used as diagnostic [https://en.wikipedia.org/wiki/Biomarker biomarkers] because it detects different mechanical forces and triggers a specific response adapted to the changes in the environment. For instance, it is sensitive to shear stress which has a major role in [https://en.wikipedia.org/wiki/Cardiovascular_physiology cardiovascular physiology]. | + | Piezo1 can be used as diagnostic [https://en.wikipedia.org/wiki/Biomarker biomarkers] because it detects different mechanical forces and triggers a specific response adapted to the changes in the environment. For instance, it is sensitive to shear stress which has a major role in [https://en.wikipedia.org/wiki/Cardiovascular_physiology cardiovascular physiology]<ref name = "Atherosclerosis"> DOI 10.2147/JIR.S319789 </ref>. |
This mechanosensitive receptor is a candidate for therapeutic innovation more specifically in the case of cardiovascular and neurodegenerative diseases. | This mechanosensitive receptor is a candidate for therapeutic innovation more specifically in the case of cardiovascular and neurodegenerative diseases. | ||
These new therapies could be based on Piezo1 pharmacological modulators. Recently, several modulators have been found. | These new therapies could be based on Piezo1 pharmacological modulators. Recently, several modulators have been found. | ||
| - | - [https://en.wikipedia.org/wiki/Receptor_antagonist Antagonists] like peptide GsMTx4 prevent the induction of [https://en.wikipedia.org/wiki/Demyelinating_disease demyelination], playing therefore a neuroprotective role. This peptide is an inhibitor of Piezo1. | + | - [https://en.wikipedia.org/wiki/Receptor_antagonist Antagonists] like peptide GsMTx4 <ref name = "GsMTx4"> DOI 10.1002/glia.23722 </ref> prevent the induction of [https://en.wikipedia.org/wiki/Demyelinating_disease demyelination], playing therefore a neuroprotective role. This peptide is an inhibitor of Piezo1. |
| - | - [https://en.wikipedia.org/wiki/Agonist Agonists] like [https://en.wikipedia.org/wiki/Yoda1 Yoda1] (a synthetic small molecule) enhance channels opening leading to demyelination and damaging the central nervous system. This molecule can activate the Piezo1 channel without mechanical stimulation. However, it can be useful to suppress the migration of transformed cells like [https://en.wikipedia.org/wiki/Fibroblast fibroblasts]. [https://en.wikipedia.org/wiki/Jedi2 Jedi2] is another chemical activator of Piezo1, but it doesn’t act on the same site as Yoda1. | + | - [https://en.wikipedia.org/wiki/Agonist Agonists] like [https://en.wikipedia.org/wiki/Yoda1 Yoda1] <ref name = "Yoda1"> DOI 10.1016/j.bbrc.2019.04.139 </ref> (a synthetic small molecule) enhance channels opening leading to demyelination and damaging the central nervous system. This molecule can activate the Piezo1 channel without mechanical stimulation. However, it can be useful to suppress the migration of transformed cells like [https://en.wikipedia.org/wiki/Fibroblast fibroblasts]. [https://en.wikipedia.org/wiki/Jedi2 Jedi2] is another chemical activator of Piezo1, but it doesn’t act on the same site as Yoda1. |
| - | - Tubeimoside 1 (TBMS1) is an inhibitor of Yoda1 allowing a decrease of the activity of Piezo 1 channels in endothelial cells. The mechanism is still unclear but according to studies, TBMS1 might be a [https://en.wikipedia.org/wiki/Competitive_inhibition competitive inhibitor], meaning that it fixes itself on the same binding site as Yoda1 and is specific to Piezo1 channels. | + | - Tubeimoside 1 (TBMS1) is an inhibitor of Yoda1 allowing a decrease of the activity of Piezo 1 channels in endothelial cells. The mechanism is still unclear but according to studies <ref name = "Tubeimoside"> DOI 10.3389/fphar.2020.00768 </ref>, TBMS1 might be a [https://en.wikipedia.org/wiki/Competitive_inhibition competitive inhibitor], meaning that it fixes itself on the same binding site as Yoda1 and is specific to Piezo1 channels. |
Discovering those modulators allows a better understanding of the mechanisms of Piezo1 channels and highlights its usefulness in the pharmacological field. | Discovering those modulators allows a better understanding of the mechanisms of Piezo1 channels and highlights its usefulness in the pharmacological field. | ||
Revision as of 14:51, 15 January 2022
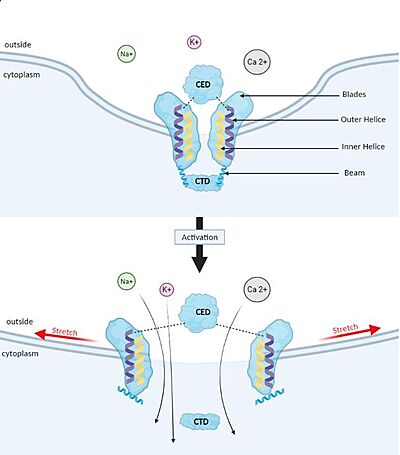
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, Zhang M, Xiao B. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron. 2016 Mar 16;89(6):1248-1263. doi: 10.1016/j.neuron.2016.01.046. Epub 2016, Feb 25. PMID:26924440 doi:http://dx.doi.org/10.1016/j.neuron.2016.01.046
- ↑ 2.0 2.1 Parpaite T, Coste B. Piezo channels. Curr Biol. 2017 Apr 3;27(7):R250-R252. doi: 10.1016/j.cub.2017.01.048. PMID:28376327 doi:http://dx.doi.org/10.1016/j.cub.2017.01.048
- ↑ 3.0 3.1 3.2 Zhou, Z. (2019). Structural Analysis of Piezo1 Ion Channel Reveals the Relationship between Amino Acid Sequence Mutations and Human Diseases. 139–155. DOI 10.4236/jbm.2019.712012
- ↑ 4.0 4.1 4.2 4.3 4.4 Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong MQ, Wang J, Li X, Xiao B. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018 Feb 22;554(7693):487-492. doi: 10.1038/nature25743. Epub 2018 Jan, 22. PMID:29469092 doi:http://dx.doi.org/10.1038/nature25743
- ↑ 5.0 5.1 5.2 5.3 Liang X, Howard J. Structural Biology: Piezo Senses Tension through Curvature. Curr Biol. 2018 Apr 23;28(8):R357-R359. doi: 10.1016/j.cub.2018.02.078. PMID:29689211 doi:http://dx.doi.org/10.1016/j.cub.2018.02.078
- ↑ 6.0 6.1 Guo YR, MacKinnon R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife. 2017 Dec 12;6. pii: 33660. doi: 10.7554/eLife.33660. PMID:29231809 doi:http://dx.doi.org/10.7554/eLife.33660
- ↑ 7.0 7.1 7.2 Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B, Yang M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015 Nov 5;527(7576):64-9. doi: 10.1038/nature15247. Epub 2015 Sep 21. PMID:26390154 doi:http://dx.doi.org/10.1038/nature15247
- ↑ 8.0 8.1 Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2017 Dec 20. pii: nature25453. doi: 10.1038/nature25453. PMID:29261642 doi:http://dx.doi.org/10.1038/nature25453
- ↑ 9.0 9.1 Lin YC, Guo YR, Miyagi A, Levring J, MacKinnon R, Scheuring S. Force-induced conformational changes in PIEZO1. Nature. 2019 Sep;573(7773):230-234. doi: 10.1038/s41586-019-1499-2. Epub 2019 Aug, 21. PMID:31435018 doi:http://dx.doi.org/10.1038/s41586-019-1499-2
- ↑ 10.0 10.1 10.2 10.3 10.4 Wei L, Mousawi F, Li D, Roger S, Li J, Yang X, Jiang LH. Adenosine Triphosphate Release and P2 Receptor Signaling in Piezo1 Channel-Dependent Mechanoregulation. Front Pharmacol. 2019 Nov 6;10:1304. doi: 10.3389/fphar.2019.01304. eCollection, 2019. PMID:31780935 doi:http://dx.doi.org/10.3389/fphar.2019.01304
- ↑ Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad KR, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature. 2014 Nov 13;515(7526):279-82. doi: 10.1038/nature13701. Epub 2014 Aug 10. PMID:25119035 doi:http://dx.doi.org/10.1038/nature13701
- ↑ Albuisson J, Murthy SE, Bandell M, Coste B, Louis-Dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, Cahalan S, Garcon L, Toutain F, Simon Rohrlich P, Delaunay J, Picard V, Jeunemaitre X, Patapoutian A. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat Commun. 2013;4:1884. doi: 10.1038/ncomms2899. PMID:23695678 doi:http://dx.doi.org/10.1038/ncomms2899
- ↑ Shinge SAU, Zhang D, Achu Muluh T, Nie Y, Yu F. Mechanosensitive Piezo1 Channel Evoked-Mechanical Signals in Atherosclerosis. J Inflamm Res. 2021 Jul 27;14:3621-3636. doi: 10.2147/JIR.S319789. eCollection, 2021. PMID:34349540 doi:http://dx.doi.org/10.2147/JIR.S319789
- ↑ Velasco-Estevez M, Gadalla KKE, Linan-Barba N, Cobb S, Dev KK, Sheridan GK. Inhibition of Piezo1 attenuates demyelination in the central nervous system. Glia. 2020 Feb;68(2):356-375. doi: 10.1002/glia.23722. Epub 2019 Oct 9. PMID:31596529 doi:http://dx.doi.org/10.1002/glia.23722
- ↑ Chubinskiy-Nadezhdin VI, Vasileva VY, Vassilieva IO, Sudarikova AV, Morachevskaya EA, Negulyaev YA. Agonist-induced Piezo1 activation suppresses migration of transformed fibroblasts. Biochem Biophys Res Commun. 2019 Jun 18;514(1):173-179. doi:, 10.1016/j.bbrc.2019.04.139. Epub 2019 Apr 24. PMID:31029419 doi:http://dx.doi.org/10.1016/j.bbrc.2019.04.139
- ↑ Liu S, Pan X, Cheng W, Deng B, He Y, Zhang L, Ning Y, Li J. Tubeimoside I Antagonizes Yoda1-Evoked Piezo1 Channel Activation. Front Pharmacol. 2020 May 25;11:768. doi: 10.3389/fphar.2020.00768. eCollection, 2020. PMID:32523536 doi:http://dx.doi.org/10.3389/fphar.2020.00768