We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1656
From Proteopedia
(Difference between revisions)
| Line 33: | Line 33: | ||
==== Catalytic domain ==== | ==== Catalytic domain ==== | ||
| - | + | The DUB family is determined by the catalytic domain. Indeed, DUBs belonging to the family of cysteine proteases have a catalytic site composed of two or three amino acids (dyads or triads). When the catalytic site is active, it may contain cysteine, histidine, aspartate or asparagine residues. As far as the metalloproteases are concerned, their active site is composed of a zinc ion and amino acids such as histidine, aspartate and serine. <ref>https://authors.library.caltech.edu/261/1/AMBpb04.pdf</ref> | |
The studied structure shows both <scene name='86/868189/Catalytic_site_polyubiquitine/2'>the catalytic site (in orange) and polyubiquitine (in purple).</scene> | The studied structure shows both <scene name='86/868189/Catalytic_site_polyubiquitine/2'>the catalytic site (in orange) and polyubiquitine (in purple).</scene> | ||
| - | Residues present in the catalytic site of DUBs are often in a '''non-functional orientation''' | + | Residues present in the catalytic site of DUBs are often in a '''non-functional orientation''' in the absence of substrate. As a result, when the substrate binds to the catalytic site of the enzyme, the site undergoes rearrangements and takes on a functional conformation. <ref>PMID:16537382</ref> The substrate opens and closes to allow the entry of the protein to be deubiquitinased. |
| - | The enzyme | + | The enzyme takes this configuration thanks to <scene name='86/868189/H_bonds_around_ser177/1'>many hydrogen bonds around Ser177.</scene> This is why phosphorylation is so important to the function of the enzyme. The phosphate group forms many links between substrate ubiquitin and a segment of the OTU domain. This is rare among the known structures of deubiquitinases. Phosphorylation-driven conformational change resembles to the one of [https://en.wikipedia.org/wiki/Kinase kinases].<ref>PMID:22245969</ref> |
== Biological role == | == Biological role == | ||
| - | + | DUBs are involved at multiple levels in the [[ubiquitin]] pathway. The modifications made by DUBs are post-translational modifications. Depending on the level, DUBs have different functions. Two specific cellular functions exist for deubiquitinases. They may act either on the degradation of the stabilization of a substrate in particular (réf 15 à ajoutées). Further functions are more specifically related to the ubiquitin molecule. Here are some of them : | |
A : '''maturation of ubiquitin'''. When ubiquitin molecules are synthesized, they are not in free form. Thus, DUBs are essential for the generation of free monomers from precursors. The degradation of precursors is carried out by several DUBs belonging to the USPs class. | A : '''maturation of ubiquitin'''. When ubiquitin molecules are synthesized, they are not in free form. Thus, DUBs are essential for the generation of free monomers from precursors. The degradation of precursors is carried out by several DUBs belonging to the USPs class. | ||
| Line 53: | Line 53: | ||
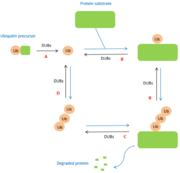
[[Image:DUBspathways.PNG |thumb|center|Figure 1 : Role of DUBs in the ubiquitin pathways]] | [[Image:DUBspathways.PNG |thumb|center|Figure 1 : Role of DUBs in the ubiquitin pathways]] | ||
| + | |||
| + | Some other deubiquitinating enzymes also have biological functions. The latter are for instance involved in growth control, transcription silencing, regulation and viral infection or even the processing of ubiquitin-like-modifications. (réf 15 à ajoutées) | ||
== Disease == | == Disease == | ||
Revision as of 13:35, 17 January 2022
| This Sandbox is Reserved from 26/11/2020, through 26/11/2021 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1643 through Sandbox Reserved 1664. |
To get started:
More help: Help:Editing |
Deubiquitinases
| |||||||||||
References
- ↑ Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007 Jan 12;315(5809):201-5. doi: 10.1126/science.1127085. PMID:17218518 doi:http://dx.doi.org/10.1126/science.1127085
- ↑ Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003 Sep 19;278(38):35857-60. doi: 10.1074/jbc.R300018200. Epub 2003, Jul 14. PMID:12860974 doi:http://dx.doi.org/10.1074/jbc.R300018200
- ↑ Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004 Nov 29;1695(1-3):189-207. doi:, 10.1016/j.bbamcr.2004.10.003. PMID:15571815 doi:http://dx.doi.org/10.1016/j.bbamcr.2004.10.003
- ↑ Urbe S, Liu H, Hayes SD, Heride C, Rigden DJ, Clague MJ. Systematic survey of deubiquitinase localization identifies USP21 as a regulator of centrosome- and microtubule-associated functions. Mol Biol Cell. 2012 Mar;23(6):1095-103. doi: 10.1091/mbc.E11-08-0668. Epub 2012, Feb 1. PMID:22298430 doi:http://dx.doi.org/10.1091/mbc.E11-08-0668
- ↑ Huang OW, Ma X, Yin J, Flinders J, Maurer T, Kayagaki N, Phung Q, Bosanac I, Arnott D, Dixit VM, Hymowitz SG, Starovasnik MA, Cochran AG. Phosphorylation-dependent activity of the deubiquitinase DUBA. Nat Struct Mol Biol. 2012 Jan 15;19(2):171-5. doi: 10.1038/nsmb.2206. PMID:22245969 doi:10.1038/nsmb.2206
- ↑ Huang OW, Ma X, Yin J, Flinders J, Maurer T, Kayagaki N, Phung Q, Bosanac I, Arnott D, Dixit VM, Hymowitz SG, Starovasnik MA, Cochran AG. Phosphorylation-dependent activity of the deubiquitinase DUBA. Nat Struct Mol Biol. 2012 Jan 15;19(2):171-5. doi: 10.1038/nsmb.2206. PMID:22245969 doi:10.1038/nsmb.2206
- ↑ https://authors.library.caltech.edu/261/1/AMBpb04.pdf
- ↑ Das C, Hoang QQ, Kreinbring CA, Luchansky SJ, Meray RK, Ray SS, Lansbury PT, Ringe D, Petsko GA. Structural basis for conformational plasticity of the Parkinson's disease-associated ubiquitin hydrolase UCH-L1. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4675-80. Epub 2006 Mar 13. PMID:16537382
- ↑ Huang OW, Ma X, Yin J, Flinders J, Maurer T, Kayagaki N, Phung Q, Bosanac I, Arnott D, Dixit VM, Hymowitz SG, Starovasnik MA, Cochran AG. Phosphorylation-dependent activity of the deubiquitinase DUBA. Nat Struct Mol Biol. 2012 Jan 15;19(2):171-5. doi: 10.1038/nsmb.2206. PMID:22245969 doi:10.1038/nsmb.2206
- ↑ Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004 Nov 29;1695(1-3):189-207. doi:, 10.1016/j.bbamcr.2004.10.003. PMID:15571815 doi:http://dx.doi.org/10.1016/j.bbamcr.2004.10.003
- ↑ Singhal S, Taylor MC, Baker RT. Deubiquitylating enzymes and disease. BMC Biochem. 2008 Oct 21;9 Suppl 1:S3. doi: 10.1186/1471-2091-9-S1-S3. PMID:19007433 doi:http://dx.doi.org/10.1186/1471-2091-9-S1-S3
- ↑ Sun J, Shi X, Mamun MAA, Gao Y. The role of deubiquitinating enzymes in gastric cancer. Oncol Lett. 2020 Jan;19(1):30-44. doi: 10.3892/ol.2019.11062. Epub 2019 Nov 7. PMID:31897112 doi:http://dx.doi.org/10.3892/ol.2019.11062
- ↑ Saldana M, VanderVorst K, Berg AL, Lee H, Carraway KL. Otubain 1: a non-canonical deubiquitinase with an emerging role in cancer. Endocr Relat Cancer. 2019 Jan 1;26(1):R1-R14. doi: 10.1530/ERC-18-0264. PMID:30400005 doi:http://dx.doi.org/10.1530/ERC-18-0264