We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1659
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
== Structure== | == Structure== | ||
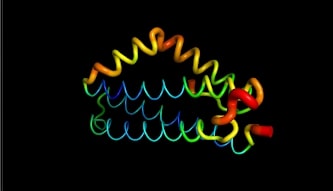
LntA is a small basic protein of 9.7 kDa. This protein is highly conserved in L. monocytogenes and is absent in almost all non-pathogenic Listeria strains . This characteristic suggests that lntA plays a key role in Listeria’s virulence. The acidic part of LntA is composed of aspartic acid (<scene name='86/868192/Acidic/1'>17,8%</scene>) and the basic part is composed of lysine and arginine (<scene name='86/868192/Basic/1'>18,6%</scene>). LntA folds in a compact helical structure and is composed of 5 alpha-helix, three of them are long antiparallel helix and can be seen as the core of the protein. The two remaining helix stick out the core. The 3 first helix are named <scene name='86/868192/Helix_h1/1'>H1</scene>, <scene name='86/868192/Helix_h2/1'>H2</scene> and <scene name='86/868192/Helix_h3/1'>H3</scene>. The 2 others are <scene name='86/868192/Helix_h4ter/1'>H4</scene> and <scene name='86/868192/Helix_h5/4'>H5</scene>. These residues located in <scene name='86/868192/These_two_helix/1'>these two helixes</scene> display high RMSD values meaning that this region is likely to oscillate. In fact, by studying this protein on pymol we can see it by the thickness and redness of these helixes which means that they have a high RMSD value : | LntA is a small basic protein of 9.7 kDa. This protein is highly conserved in L. monocytogenes and is absent in almost all non-pathogenic Listeria strains . This characteristic suggests that lntA plays a key role in Listeria’s virulence. The acidic part of LntA is composed of aspartic acid (<scene name='86/868192/Acidic/1'>17,8%</scene>) and the basic part is composed of lysine and arginine (<scene name='86/868192/Basic/1'>18,6%</scene>). LntA folds in a compact helical structure and is composed of 5 alpha-helix, three of them are long antiparallel helix and can be seen as the core of the protein. The two remaining helix stick out the core. The 3 first helix are named <scene name='86/868192/Helix_h1/1'>H1</scene>, <scene name='86/868192/Helix_h2/1'>H2</scene> and <scene name='86/868192/Helix_h3/1'>H3</scene>. The 2 others are <scene name='86/868192/Helix_h4ter/1'>H4</scene> and <scene name='86/868192/Helix_h5/4'>H5</scene>. These residues located in <scene name='86/868192/These_two_helix/1'>these two helixes</scene> display high RMSD values meaning that this region is likely to oscillate. In fact, by studying this protein on pymol we can see it by the thickness and redness of these helixes which means that they have a high RMSD value : | ||
| + | |||
[[Image:pymol1.jpg]] | [[Image:pymol1.jpg]] | ||
Current revision
| |||||||||||
References
- ↑ ROHDE JOHN R. Listeria unwinds host’s DNA. SCIENCE, 2011 : 1271-1272
- ↑ Winter SE, Thiennimitr P et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010
- ↑ Dewoody, R., Merritt, P.M., Houppert, A.S. and Marketon, M.M. (2011), YopK regulates the Yersinia pestis type III secretion system from within host cells. Molecular Microbiology, 79: 1445-1461. https://doi.org/10.1111/j.1365-2958.2011.07534.x
- ↑ Lebreton A, Job V, Ragon M, Le Monnier A, Dessen A, Cossart P, Bierne H. 2014. Structural basis for the inhibition of the chromatin repressor BAHD1 by the bacterial nucleomodulin LntA
- ↑ Alice Lebreton. Régulations post-transcriptionnelles de l’expression génique de la cellule hôte en réponse à l’infection bactérienne. Sciences du Vivant, 2015