Sandbox Reserved 1700
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
=== Transmembrane Domain === | === Transmembrane Domain === | ||
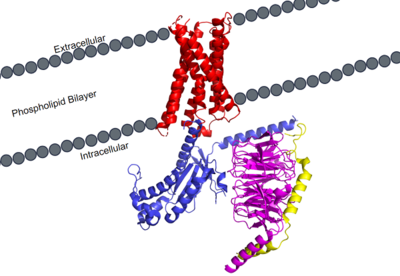
The transmembrane domain spans the cellular membrane. It consists of <scene name='90/904305/Transmembrane_protein_c_and_l/1'>seven transmembrane helices</scene>, three extracellular loops, and three intracellular loops. The transmembrane helices are numbered 1-7 and contain special conserved motifs that are shared across other A family receptors. These motifs are expanded upon later, as they heavily contribute to the structure and therefore function of the transmembrane domain as a whole. | The transmembrane domain spans the cellular membrane. It consists of <scene name='90/904305/Transmembrane_protein_c_and_l/1'>seven transmembrane helices</scene>, three extracellular loops, and three intracellular loops. The transmembrane helices are numbered 1-7 and contain special conserved motifs that are shared across other A family receptors. These motifs are expanded upon later, as they heavily contribute to the structure and therefore function of the transmembrane domain as a whole. | ||
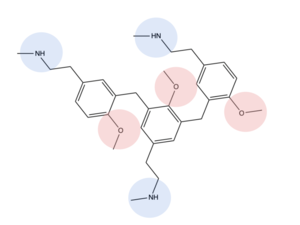
| - | The extracellular domain of the protein is responsible for ligand binding, which initiates signal transduction. The properties of the extracellular environment of the transmembrane domain determines what ligands bind to the protein, and what type of binding interactions the protein and ligand have. In MRGPRX2, the extracellular side of the protein has two | + | The extracellular domain of the protein is responsible for ligand binding, which initiates signal transduction. The properties of the extracellular environment of the transmembrane domain determines what ligands bind to the protein, and what type of binding interactions the protein and ligand have. In MRGPRX2, the extracellular side of the protein has one binding pocket with two sub-pockets. Sub-pocket 1is positively charged due to positively charged lysine residues, while sub-pocket 2 contains hydrophobic amino acids, which contribute to hydrophobic interactions between ligand and protein. |
The intracellular domain is what connects the transmembrane and G-protein domains together. There are a wide variety of residues and important interactions that contribute to this interaction, and it is very important in transmitting the extracellular signal of ligand binding to the intracellular environment where the G-protein binds and can become activated. | The intracellular domain is what connects the transmembrane and G-protein domains together. There are a wide variety of residues and important interactions that contribute to this interaction, and it is very important in transmitting the extracellular signal of ligand binding to the intracellular environment where the G-protein binds and can become activated. | ||
Revision as of 19:44, 27 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
MRGPRX2 Human Itch G-Protein Coupled Receptor (GPCR)
| |||||||||||
References
- ↑ Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017 Dec;16(12):829-842. doi: 10.1038/nrd.2017.178. Epub, 2017 Oct 27. PMID:29075003 doi:http://dx.doi.org/10.1038/nrd.2017.178
- ↑ Basith S, Cui M, Macalino SJY, Park J, Clavio NAB, Kang S, Choi S. Exploring G Protein-Coupled Receptors (GPCRs) Ligand Space via Cheminformatics Approaches: Impact on Rational Drug Design. Front Pharmacol. 2018 Mar 9;9:128. doi: 10.3389/fphar.2018.00128. eCollection, 2018. PMID:29593527 doi:http://dx.doi.org/10.3389/fphar.2018.00128
- ↑ 3.0 3.1 Cao C, Kang HJ, Singh I, Chen H, Zhang C, Ye W, Hayes BW, Liu J, Gumpper RH, Bender BJ, Slocum ST, Krumm BE, Lansu K, McCorvy JD, Kroeze WK, English JG, DiBerto JF, Olsen RHJ, Huang XP, Zhang S, Liu Y, Kim K, Karpiak J, Jan LY, Abraham SN, Jin J, Shoichet BK, Fay JF, Roth BL. Structure, function and pharmacology of human itch GPCRs. Nature. 2021 Dec;600(7887):170-175. doi: 10.1038/s41586-021-04126-6. Epub 2021, Nov 17. PMID:34789874 doi:http://dx.doi.org/10.1038/s41586-021-04126-6
- ↑ 4.0 4.1 Yang F, Guo L, Li Y, Wang G, Wang J, Zhang C, Fang GX, Chen X, Liu L, Yan X, Liu Q, Qu C, Xu Y, Xiao P, Zhu Z, Li Z, Zhou J, Yu X, Gao N, Sun JP. Structure, function and pharmacology of human itch receptor complexes. Nature. 2021 Dec;600(7887):164-169. doi: 10.1038/s41586-021-04077-y. Epub 2021, Nov 17. PMID:34789875 doi:http://dx.doi.org/10.1038/s41586-021-04077-y