We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1726
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
After binding to one of its ligands, Anaplastic Lymphoma Kinase undergoes <scene name='90/904331/Alk_full_dimerization/1'>ligand-induced dimerization</scene> <ref name="Huang">PMID:30400214</ref>. The dimerization causes trans-phosphorylation of specific tyrosine residues which in turn amplifies the signal. It has been presumed that the phosphorylation cascade activates ALK kinase activity <ref name="Huang" />. | After binding to one of its ligands, Anaplastic Lymphoma Kinase undergoes <scene name='90/904331/Alk_full_dimerization/1'>ligand-induced dimerization</scene> <ref name="Huang">PMID:30400214</ref>. The dimerization causes trans-phosphorylation of specific tyrosine residues which in turn amplifies the signal. It has been presumed that the phosphorylation cascade activates ALK kinase activity <ref name="Huang" />. | ||
== Function == | == Function == | ||
| - | + | Anaplastic Lymphoma Kinase plays a role in cellular communication and in the normal development and function of the nervous system <ref name="Morris">PMID:9174053<ref/>. | |
== Disease and Medical Relevance == | == Disease and Medical Relevance == | ||
=== Cancer === | === Cancer === | ||
Revision as of 20:25, 29 March 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
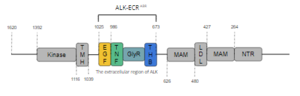
Anaplastic Lymphoma Kinase Extracellular Region
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ Borenas M, Umapathy G, Lai WY, Lind DE, Witek B, Guan J, Mendoza-Garcia P, Masudi T, Claeys A, Chuang TP, El Wakil A, Arefin B, Fransson S, Koster J, Johansson M, Gaarder J, Van den Eynden J, Hallberg B, Palmer RH. ALK ligand ALKAL2 potentiates MYCN-driven neuroblastoma in the absence of ALK mutation. EMBO J. 2021 Feb 1;40(3):e105784. doi: 10.15252/embj.2020105784. Epub 2021 Jan 7. PMID:33411331 doi:http://dx.doi.org/10.15252/embj.2020105784
- ↑ 3.0 3.1 Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ PMID:9174053<ref></ref>.
Disease and Medical Relevance
Cancer
In ALK fusion proteins, the ALK fusion partner may cause dimerization independent of ligand binding, causing oncogenic ALK activation <ref></ref>.
The regulation of ALK dimerization by ALKAL points to clear ways to inhibit ALK activity and may offer new therapeutic strategies in multiple disease settings <ref>PMID:34819665</li></ol></ref>