Introduction
G protein-coupled receptors (GPCRs) are the largest class of integral membrane proteins.[1] GPCRs[1][2] are divided into five families; the rhodopsin family (class A), the secretin family (class B), the glutamate family (class C), the frizzled/taste family (class F), and the Adhesion G protein-coupled receptor| adhesion family.[1][3] All GPCRs contain a similar seven α-helical transmembrane domain that undergoes a conformation change once bound to its ligand. This conformational change then transduces a signal to a coupled, heterotrimeric G protein which then dictates whether an intracellular signaling pathway will be initiated or inhibited. The initiation of the intracellular signaling pathway occurs in response to a variety of stimuli such as light, Ca2+, peptides, different proteins, and many more stimuli. Ultimately, intracellular signaling accomplishes many interesting physiological roles accomplishes many interesting physiological roles.[1][3]
Human Itch G-coupled protein receptors (GPCRs), or Mast cell-related GPCRs (MRGPRX), have been identified as pruritogenic receptors and are found in human sensory neurons, specifically in the connective tissue mast cells and dorsal root ganglia in humans.[4]
The determination of the first structures of a ligand-activated GPCR was achieved by Robert J. Lefkowitz and Brian K. Kobilka which won them the 2012 Nobel Prize in Chemistry. They also successfully captured images of the first activated GPCR in a complex with a G protein. See Nobel Prizes for 3D Molecular Structure.
Related Enzymes
Among Human Itch GPCRs, MRGPRX2 is a class A GPCR that regulates mast cell degranulation and itch-related hypersensitivity reactions.[5][6] MRGPRX2 is also a target of morphinan alkaloids, like morphine, codeine, and dextromethorphan.[5][6] MRGPRX2 couples to nearly all G-protein families and subtypes with robust coupling to Gq and Gi families.[5][6]
is another sub-group of the MRGPRX family, which mediates cholestatic itch and is a target of nateglinide drugs.[5][6] MRGPRX4 also couples to Gq and Gi similar to MRGPRX2.
Structure Overview
The contains 7 transmembrane helices (TM 1-7), 3 intracellular loops (ICL 1-3), and 3 extracellular loops (ECL 1-3).[5] Ligand binding occurs at the N-terminus in the extracellular domain and is composed of two binding sub-pockets.[5] The intracellular domain consists of helix VII and a C-terminal sequence, which binds the G-protein and promotes downstream signaling.[5]
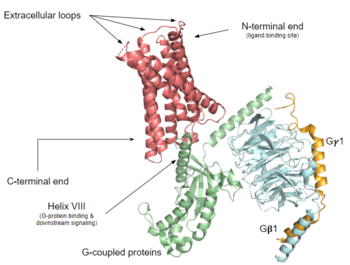
Structural overview of MRGPRX2
G-proteins and Signaling
G-proteins, when paired with a GPCR, assist in signal transduction.[7][8] G-proteins are heterotrimeric GTPases composed of three subunits: . The α subunit acts as the main signal mediator and contains a binding site for GDP or GTP, which acts as a biological “switch” to regulate the transmission of a signal from the activated receptor.[7][8] The α subunit will dissociate and can then move in the plane of the membrane from the receptor to bind to downstream effectors to continue signal transmission and ultimately produce a cellular response.[7][8]
Gq and Gi Family α Subunits
The actions that G-proteins induce can be classified based on the sequence homology of α subunit (Gα) present.[7][9] The most well-known are referred to as Gi, Gs, and Gq. The Gs and Gq proteins are stimulatory, while the Gi protein is inhibitory.[7][9] In addition, proteins are classified based on the signaling pathway that they regulate. For example, Gq proteins are seen in a signaling pathway that relies on phospholipase C enzymes, while Gs and Gi proteins are regulators of adenylate cyclase.[9] On this page, we will be focusing solely on the structures of the Gq and Gi proteins and their interactions with mast-cell receptors.
Gαq and Gαi are proteins comprised of 359 amino acid residues, with varying sequences, that both contain a helical domain and a GTPase binding domain.[9] The GTPase binding domain is responsible for the hydrolysis of GTP as well as the binding of the β and γ subunits that form the trimeric protein structure. The helical domain contains six α helices, which are responsible for the binding of the G-protein to the coupled receptor.[9]
The conformations of the G-proteins vary based on their association with a particular membrane receptor due to interactions between the amino acids in the N-terminus of the α subunit and the C-terminus of the receptor.[9]
Ligand Binding Site
Sub-pocket 1
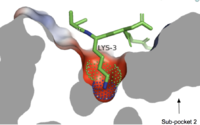
Cross-sectional view of electrostatic surface of MRPRX2 sub-pocket 1 interaction with lysine 3 of cortistatin-1
Sub-pocket 1 is formed by TM3, TM6, and ECL2.[5] This sub-pocket is both small and deep which results in the binding of only a single amino acid residue, namely arginine or sometimes lysine.[5] The binding is mediated by two protein within the binding site: Glu164 and Asp184.[5] The strong charge interactions of these two residues create a highly negatively charged electrostatic interaction within this sub-pocket.[5]
Sub-pocket 2
Sub pocket 2 is formed by TM1, TM2, TM6, and TM7.[5] This binding sub-pocket is much more shallow and allows for the binding of larger structures.[5] The key residues involved are Trp243 and Phe170 which allow for binding through hydrophobic interactions.[5] The hydrophobicity of this binding pocket accounts for the large electrostatic difference observed between the two sub pockets.
Differences To Most Class A GPCRs
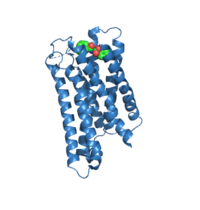
A. MRGPRX2 (7S8P) depicting the depth in which the ligand interacts within the receptor
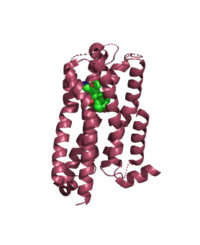
B. 5HT2A (6WHA) - class A GPCR
The unique characteristics of in comparison to class A GPCRs provides an explanation for the differences in ligand interactions. These differences in intermolecular interactions and structural motifs contribute to the surface level ligand binding in MRGPRX2, whereas the typical ligand interaction occurs deep within the helices in class A GPCRs
Toggle Switch
The of class A GPCRs enables the receptor to initiate the signaling cascade. However, MRGPRX2 does not contain the conserved ‘toggle switch’ Trp. Instead, it is replaced by Gly.[5] Therefore, the main residues of this motif in MRGPRX2 are Gly236, Tyr113, Phe239, and Trp243.[5] As a result, TM6 is shifted closer to TM3 on the extracellular side of the membrane. This conformational change may account for the lack of ligand binding of MRGPRX2 as compared to family A receptors.[5] This toggle switch swap also means that ligands, such as (R)-zinc-3573 and Cortistatin-14, bind in a different spot than ligands do on other class A GPCRs. In MRGPRX2, Gly236 is located closer to the bottom of the interface, which is the same in MRGPRX4 (Gly229). Compared with other structures, such as 5-HT2AR, A2AR, and β2AR, the TM6 helices of MRGPRX2 and MRGPRX4 are closer to the TM3 helix which makes the binding pocket more occluded than seen in canonical structures.[5]
PIF/LLF motif
The MRGPRX2 structure does not contain the conserved at the TM3-TM6 interface.[5] Canonically, the PIF motif consists of a Pro, Ile, and Phe which transduce the signal produce by ligand binding through the TMD within conserved distances (5.50Å, 3.40Å, and 6.44Å respectively).[5][10] In this motif, the residues are not conserved at specific positions in the amino acid sequence but instead are conserved at distances that allow them to interact.[5]
In MRGPRX2, the residues that make up TM5 have shifted down two residues making Leu194 analogous to the position of the Pro in other GPCRs. However, in MRGPRX2, Leu194 is slightly closer to the other residues in the motif at 5.48Å.[5] The residue at a distance of 5.50Å in MRGPRX2 is Met196. It does not interact with the motif because it is angled away from the TM3 and TM6 interface.[5] Leu194 interacts with two other residues, Leu117 and Phe232. The additional change from Ileto Leu is why the motif in MRGPRX2 is called the LLF motif.[5] Compared with other structures, such as 6WHA| 5-HT2AR, 5G53| A2AR, and link 3SN6| β2AR, the TM6 helices of MRGPRX2 are closer to the TM3 helix due to the shift in residues which makes the binding pocket more occluded than seen in canonical structures.[5]
DRY/ERC motif
A (residues 130-132) is present in all GPCRs, while the majority of class A GPCRs have a conserved E/DRY motif. However, MRGPRX2 contains an , which replaces Tyr174 with Cys128.[5] This replacement alters the spatial organization of the helices due to the fact that Tyr is no longer present to push the helices outward.[5] As a result, the binding site in MRGPRX2 is shallower which lead to surface-level ligand interactions.[5]
Disulfide bonds
In general, class A GPCRs have a conserved disulfide bond between TM3 and ECL2.[5] In contrast, the is located between Cys168 of TM4 and Cys180 of TM5, which structurally flips ECL2 to the top of TM4 and TM5.[5] This creates the wide ligand-binding surface of MRGPRX2 that contributes to surface level binding which allows diverse ligand interactions.[5]
Sodium binding site
The of MRGPRX2 contains the conserved Asp75, however, the conserved Ser116 on TM3 seen in class A GPCRs is replaced by Gly116.[5] The presence of Gly as opposed to the polar Ser contributes to a lack of polar character in addition to decreasing the size of the binding pocket. The sodium pocket generally serves as an allosteric binding pocket for sodium. Both factors thereby limit the binding of sodium ions to the sodium binding site in MRGPRX2.
Mechanism
Binding to the extracellular N-terminus domain triggers a transmembrane conformation change of MRGPRX2, which demonstrates a less significant change when compared to other class A GPCRs due to the surface level binding of the ligand to MRGPRX2.[5] Once ligand binding and the conformational change to the active state have taken place, the signal is relayed to the α-subunit of the heterotrimeric G-protein.[8] The α-subunit will then exchange a GDP for GTP to initiate the dissociation of the α, β, and γ subunits.[8] During this dissociation, the α-subunit is able to travel away from the receptor in the plane of the membrane to bind to downstream effectors to produce a cellular response.[8]
Ligand interactions
-
- C48/80 is a peptide agonist that can bind to MRGPRX2 when it is associated with a Gi or Gq protein.[6] The binding interactions of C48/80 with MRGPRX2 do not change whether the GPCR is associated with Gi and Gq, but the binding affinity of C48/80 to MRGPRX2 is different.[6] The structure of the ligand itself consists of three phenethylamine groups that are arranged in a Y shape with a semicircular arrangement.[6] Upon its binding, the Asp184 and Glu164 within sub-pocket 1 interact only with the central phenethylamine ring, forming hydrogen bonds and charge-charge interactions.[6] The central phenethylamine ring is inserted into the binding pocket at a depth of 5.6Å. This binding depth means that it is further from the toggle switch (17.7Å) than structures such as Cortistatin-14 (13.7Å).[6] The binding of this ligand induces a conformational change that causes the separation of all of the ECLs from the receptor's N-terminus.[6]
-
- (R)-zinc-3573 is an agonist that binds to MRGPRX2 when it is associated with a Gi or Gq protein.[5] This agonist is a small cationic molecule that forms largely ionic interactions with the negatively-charged sub-pocket 1 and has no interactions with sub-pocket 2.[5] (R)-zinc-3573 forms hydrogen bonds and hydrophobic interactions with Asp184 and Glu164 of sub-pocket 1.[5] (R)-zinc-3573 was identified through chemical screening as a MRGPRX2-selective agonist and was used to study MRGPRX2-mediated mast cell proliferation, MRGPRX2 receptor expression, mediator release and inhibition, and signaling.[11]
-
- Cortistatin-14 is an endogenous, cyclic, neuropeptide agonist which interacts with MRGPRX2 in the same way whether it is coupled to Gi or Gq proteins.[5][12] Cortistation-14 is widely available in many systems throughout the body and naturally functions to regulate many physiological and pathological mechanisms. These mechanisms include, but are not limited to reducing locomotion, inducing sleep, inhibiting neuronal activity, and inhibiting cell proliferation.[12] Cortistatin-14 is a fairly large ligand and it binds near the ECLs in subpocket-1.[5] Specifically, a lysine residue (Lys3) on Cortistatin-14 binds in the negatively-charged sub-pocket 1 and forms strong charge interactions with Asp184 and Glu164.[5] The remaining residues of Cortistatin-14 will extend over to sub-pocket 2 and bind through hydrophobic interactions.[5]
Clinical Relevance
MRGPRX2 initiates IgE-mediated anaphylactic reactions.[13] MRGPRX2-mediated anaphylactic responses occur more quickly than IgE-mediated responses, but the responses also tended to be more transient.[13] Common commercial drugs, like icatibant and cetrorelix, as well as neuromuscular blocking agents activate mast cells through the MRGPRX2 pathway.
Many mutations also affect the actions of MRGPRX2. For example, a single residue mutation in sub-pocket 1 (Glu164Arg) prevented interactions between the receptor and ligands like C48/80.[13] In addition, single nucleotide polymorphisms (SNPs) have been linked to many variations of MRGPRX2 which predispose patients to hyperactivation of the receptors. Two of the most common SNPs are Asn62Thr which affects the cytoplasmic domain and Asn16His which affects the extracellular domain.[13] These mutations have been theorized to potentially protect patients from drug-induced mast cell degranulation and hypersensitivity reactions.
Interestingly, within the MRGPRX2-mediated pathway, reaction frequencies differ. In the same patients, anaphylactic reactions can occur by both IgE-mediated and MRGPRX2-mediated pathways. The presence of both mechanisms within a patient may be responsible for cross-reactivity between drugs.[13] This has led to a hypothesis that certain drugs may interact with MRGPRX2 on different active sites within the receptor. In addition, studies have also led to hypotheses that the intracellular signaling pathways triggered by the binding of varying drugs may have different intracellular responses based on the site of binding.
See Also
References
- ↑ 1.0 1.1 1.2 1.3 Zhang D, Zhao Q, Wu B. Structural Studies of G Protein-Coupled Receptors. Mol Cells. 2015 Oct;38(10):836-42. doi: 10.14348/molcells.2015.0263. Epub 2015, Oct 15. PMID:26467290 doi:http://dx.doi.org/10.14348/molcells.2015.0263
- ↑ Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010 Jan;24(1):261-74. Epub 2009 Dec 17. PMID:20019124 doi:10.1210/me.2009-0473
- ↑ 3.0 3.1 Zhang Y, Devries ME, Skolnick J. Structure modeling of all identified G protein-coupled receptors in the human genome. PLoS Comput Biol. 2006 Feb;2(2):e13. Epub 2006 Feb 17. PMID:16485037 doi:http://dx.doi.org/10.1371/journal.pcbi.0020013
- ↑ doi: https://dx.doi.org/10.1016/j.tins.2010.09.002<ref></ref>TheyareclassifiedasclassAGPCRs,however,MRGPRXreceptorsrespondtoadiversenumberofagonists,antagonists,andinverseagonistssomeofwhicharenottypicalligandsofclassAreceptors.MRGPRXareinvolvedinhostdefense,pseudo-allergicreactions,non-histaminergicitch,periodontitis,neurogenicinflammation,andinflammatorypain.<refname="davidson2011"
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 5.23 5.24 5.25 5.26 5.27 5.28 5.29 5.30 5.31 5.32 5.33 5.34 5.35 5.36 5.37 5.38 5.39 Cao C, Kang HJ, Singh I, Chen H, Zhang C, Ye W, Hayes BW, Liu J, Gumpper RH, Bender BJ, Slocum ST, Krumm BE, Lansu K, McCorvy JD, Kroeze WK, English JG, DiBerto JF, Olsen RHJ, Huang XP, Zhang S, Liu Y, Kim K, Karpiak J, Jan LY, Abraham SN, Jin J, Shoichet BK, Fay JF, Roth BL. Structure, function and pharmacology of human itch GPCRs. Nature. 2021 Dec;600(7887):170-175. doi: 10.1038/s41586-021-04126-6. Epub 2021, Nov 17. PMID:34789874 doi:http://dx.doi.org/10.1038/s41586-021-04126-6
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 6.9 Yang F, Guo L, Li Y, Wang G, Wang J, Zhang C, Fang GX, Chen X, Liu L, Yan X, Liu Q, Qu C, Xu Y, Xiao P, Zhu Z, Li Z, Zhou J, Yu X, Gao N, Sun JP. Structure, function and pharmacology of human itch receptor complexes. Nature. 2021 Dec;600(7887):164-169. doi: 10.1038/s41586-021-04077-y. Epub 2021, Nov 17. PMID:34789875 doi:http://dx.doi.org/10.1038/s41586-021-04077-y
- ↑ 7.0 7.1 7.2 7.3 7.4 Edward Zhou X, Melcher K, Eric Xu H. Structural biology of G protein-coupled receptor signaling complexes. Protein Sci. 2019 Mar;28(3):487-501. doi: 10.1002/pro.3526. Epub 2018 Dec 13. PMID:30311978 doi:http://dx.doi.org/10.1002/pro.3526
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Nelson, David L. (David Lee), 1942-. (2005). Lehninger principles of biochemistry. New York :W.H. Freeman
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Kamato D, Thach L, Bernard R, Chan V, Zheng W, Kaur H, Brimble M, Osman N, Little PJ. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Galpha/q,11. Front Cardiovasc Med. 2015 Mar 24;2:14. doi: 10.3389/fcvm.2015.00014. eCollection, 2015. PMID:26664886 doi:http://dx.doi.org/10.3389/fcvm.2015.00014
- ↑ Schonegge AM, Gallion J, Picard LP, Wilkins AD, Le Gouill C, Audet M, Stallaert W, Lohse MJ, Kimmel M, Lichtarge O, Bouvier M. Evolutionary action and structural basis of the allosteric switch controlling beta2AR functional selectivity. Nat Commun. 2017 Dec 18;8(1):2169. doi: 10.1038/s41467-017-02257-x. PMID:29255305 doi:http://dx.doi.org/10.1038/s41467-017-02257-x
- ↑ Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, Che T, Nagase H, Carroll FI, Jin J, Shoichet BK, Roth BL. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol. 2017 May;13(5):529-536. doi: 10.1038/nchembio.2334. Epub 2017 Mar , 13. PMID:28288109 doi:http://dx.doi.org/10.1038/nchembio.2334
- ↑ 12.0 12.1 Jiang J, Peng Y, Liang X, Li S, Chang X, Li L, Chang M. Centrally Administered Cortistation-14 Induces Antidepressant-Like Effects in Mice via Mediating Ghrelin and GABAA Receptor Signaling Pathway. Front Pharmacol. 2018 Jul 19;9:767. doi: 10.3389/fphar.2018.00767. eCollection, 2018. PMID:30072893 doi:http://dx.doi.org/10.3389/fphar.2018.00767
- ↑ 13.0 13.1 13.2 13.3 13.4 Porebski G, Kwiecien K, Pawica M, Kwitniewski M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front Immunol. 2018 Dec 20;9:3027. doi: 10.3389/fimmu.2018.03027. eCollection, 2018. PMID:30619367 doi:http://dx.doi.org/10.3389/fimmu.2018.03027




