Methionine synthase
From Proteopedia
| Line 7: | Line 7: | ||
[[Image:Overall.jpeg]] | [[Image:Overall.jpeg]] | ||
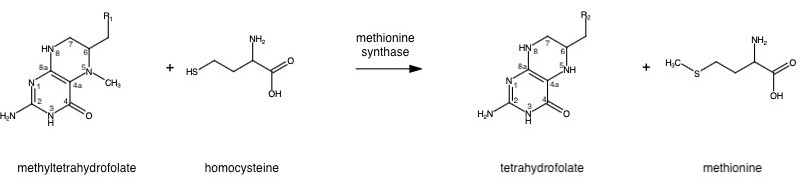
| - | The change from homocysteine to methionine is an SN2 reaction, as seen above, where the methyl group on N-5 from methyltetrahydrofolate (MTHF), is donated. MTHF is a product of Methylenetetrahydrofolate reductase (MTHFR) from the folate cycle [link Shaylie's page here]. This is a complex reaction as tetrahydrofolate, the product, is a poor leaving group, thus requiring a "super nucleophile", vitamin B12 cob(I)alamin, to carry out the reaction<ref name="Kung et al">DOI: 10.1038/nature10916</ref>. | + | The change from homocysteine to methionine is an SN2 reaction, as seen above, where the methyl group on N-5 from methyltetrahydrofolate (MTHF), is donated. MTHF is a product of Methylenetetrahydrofolate reductase (MTHFR) from the folate cycle [link Shaylie's page here]. This is a complex reaction as tetrahydrofolate (THF), the product, is a poor leaving group, thus requiring a "super nucleophile", vitamin B12 cob(I)alamin, to carry out the reaction<ref name="Kung et al">DOI: 10.1038/nature10916</ref>. |
== Relevance == | == Relevance == | ||
| Line 17: | Line 17: | ||
== Structural highlights == | == Structural highlights == | ||
| - | The full structure of MS has yet to be determined but studies have found it contains <scene name='90/907471/Superposition_1/2'>4 domains</scene>, each domain with a unique function that bind to Cob(I)alamin as the methyl carrier (in pink), methyltetrahydrofolate as the methyl donor in the catalytic cycle (in blue), Homocysteine as the methyl acceptor (in yellow), and S-adenosyl-L-methionine or SAM (in red) as the methyl donor in the reactivation cycle<ref name="Bandarian et al">DOI: 10.1038/nsb738</ref>. During each cycle, the domains must be positioned close enough to the Cobalamin | + | The full structure of MS has yet to be determined but studies have found it contains <scene name='90/907471/Superposition_1/2'>4 domains</scene>, each domain with a unique function that bind to Cob(I)alamin as the methyl carrier (in pink), methyltetrahydrofolate as the methyl donor in the catalytic cycle (in blue), Homocysteine as the methyl acceptor (in yellow), and S-adenosyl-L-methionine or SAM (in red) as the methyl donor in the reactivation cycle<ref name="Bandarian et al">DOI: 10.1038/nsb738</ref>. During each cycle, the domains must be positioned close enough to the Cobalamin to allow for methyl transfer to occur. |
== Vitamin B12 == | == Vitamin B12 == | ||
| - | PDB ID: 1K7Y | + | PDB ID: 1K7Y, the B12 domain of MS. |
[[Image:cob(I)alamin.jpg]] | [[Image:cob(I)alamin.jpg]] | ||
Revision as of 14:09, 13 April 2022
This page is being worked on during the Spring 2022 semester.
Function
Methionine is an essential amino acid required by our bodies for healthy cell and tissue growth. It is essential because is not naturally derived, and must be obtained from our diet first in the form of homocysteine. Methionine synthase (abbrev. MS; EC: 2.1.1.13), a B12-dependent enzyme, is a critical part of the one-carbon metabolism cycle because it converts homocysteine to methionine.
The change from homocysteine to methionine is an SN2 reaction, as seen above, where the methyl group on N-5 from methyltetrahydrofolate (MTHF), is donated. MTHF is a product of Methylenetetrahydrofolate reductase (MTHFR) from the folate cycle [link Shaylie's page here]. This is a complex reaction as tetrahydrofolate (THF), the product, is a poor leaving group, thus requiring a "super nucleophile", vitamin B12 cob(I)alamin, to carry out the reaction[1].
Relevance
Methionine deficiency can result in diseases such as birth abnormalities[1].
| |||||||||||
References
- ↑ 1.0 1.1 Kung Y, Ando N, Doukov TI, Blasiak LC, Bender G, Seravalli J, Ragsdale SW, Drennan CL. Visualizing molecular juggling within a B(12)-dependent methyltransferase complex. Nature. 2012 Mar 14. doi: 10.1038/nature10916. PMID:22419154 doi:10.1038/nature10916
- ↑ 2.0 2.1 Bandarian V, Pattridge KA, Lennon BW, Huddler DP, Matthews RG, Ludwig ML. Domain alternation switches B(12)-dependent methionine synthase to the activation conformation. Nat Struct Biol. 2002 Jan;9(1):53-6. PMID:11731805 doi:10.1038/nsb738
- ↑ Barra L, Fontenelle C, Ermel G, Trautwetter A, Walker GC, Blanco C. Interrelations between glycine betaine catabolism and methionine biosynthesis in Sinorhizobium meliloti strain 102F34. J Bacteriol. 2006 Oct;188(20):7195-204. doi: 10.1128/JB.00208-06. PMID:17015658 doi:http://dx.doi.org/10.1128/JB.00208-06
- ↑ doi: https://dx.doi.org/10.1073/pnas.0906132106
Proteopedia Page Contributors and Editors (what is this?)
Kia Yang, Karsten Theis, Michal Harel, Anna Postnikova, Michael O'Shaughnessy