User:George G. Papadeas/Sandbox VKOR
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
== Structural Highlights== | == Structural Highlights== | ||
===Structural Overview=== | ===Structural Overview=== | ||
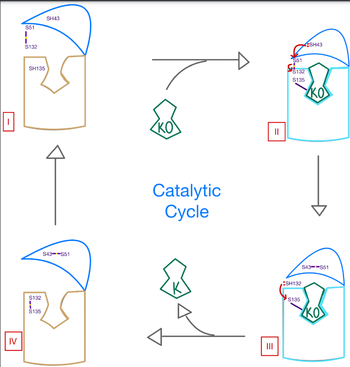
| - | VKOR has many key components of its structure that allow it to maintain proper functionality and catalytic abilities. The VKOR | + | VKOR has many key components of its structure that allow it to maintain proper functionality and catalytic abilities. The VKOR binding pocket allows for specific substrate binding via many highly conserved residues that can recognize the target substrates. The pocket works in conjunction with the cap domain, which is a helical component of the VKOR that facilitates the conformation from the open to closed conformation of the enzyme once the substrate binds. Interactions between the cap domain, the active site, and the bound protein are critical to achieve full activation of Vitamin K. Another important part of the structure is the anchor, which simply serves as a way to hold VKOR within the proper orientation in the cell membrane such that all enzymatic components are in correct proximity for substrate binding and catalysis. |
| + | Vital to the VKOR structure are two disulfide bridges. The first appears slightly above the binding pocket between C132 and C135. The second occurs within the cap domain between C43 and C51. These cysteines are catalytic residues that aid in the transition of VKOR from the <scene name='90/906893/Open_conformation/1'>open conformation</scene> to the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene> and the reduction of KO. | ||
=== Active Site === | === Active Site === | ||
| - | Within the four transmembrane helices lies the <scene name='90/906893/Active_site/4'> | + | Within the four transmembrane helices lies the <scene name='90/906893/Active_site/4'>binding pocket</scene>. The active site is comprised of a hydrophobic pocket containing two hydrophilic residues, N80 and Y139, that interact with substrates and ligands alike. The hydrophobic pocket provides specificity to the region while the hydrophilic residues hydrogen bond to the substrate, providing recognition and increasing specificity. The C132-C135 disulfide bridge above the binding pocket provides stabilization when a substrate is bound. This bridge provides increased stability for the binding site as it interacts with and binds substrates or inhibitors. Upon binding, VKOR will transition into the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene> allowing the catalytic mechanism to commence. |
| - | + | ||
| - | + | ||
=== Cap Domain === | === Cap Domain === | ||
Revision as of 15:00, 13 April 2022
VKOR
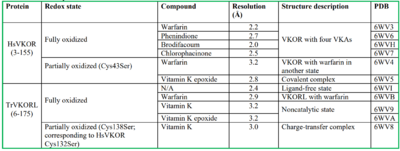
| |||||||||||
References
1. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
2. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Unknown PubmedID 10.1126
- ↑ Unknown PubmedID 10.1126