We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Thymidylate synthase
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Structural highlights == | == Structural highlights == | ||
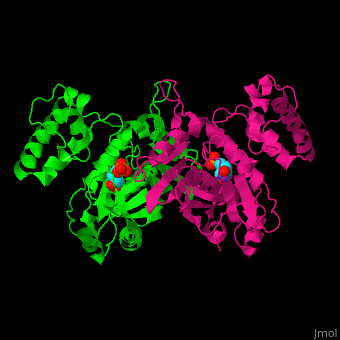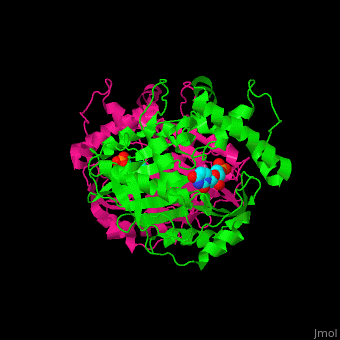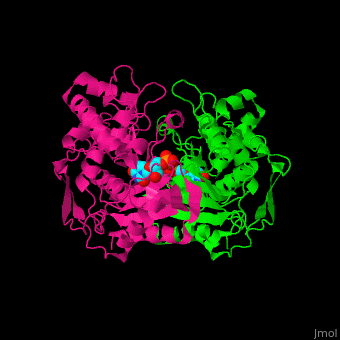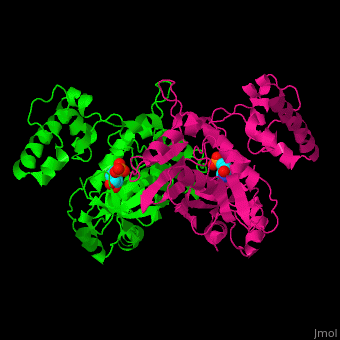
TS <scene name='49/493689/Cv/4'>active site contains the substrate dUMP</scene><ref>PMID:9053905</ref>. Water molecules are shown as red spheres. | TS <scene name='49/493689/Cv/4'>active site contains the substrate dUMP</scene><ref>PMID:9053905</ref>. Water molecules are shown as red spheres. | ||
| + | |||
The active site Cysteine has two <scene name='90/907470/Ts_7jxf_percentb/3'>conformations</scene>. | The active site Cysteine has two <scene name='90/907470/Ts_7jxf_percentb/3'>conformations</scene>. | ||
<jmol> | <jmol> | ||
Revision as of 15:07, 13 April 2022
Thymidylate Synthase (TS) is an important enzyme in one-carbon metabolism[1]. TS catalyzes the transfer of a methyl group and a hydride from 5,10-methylenetetrahydrofolate to 2-deoxyuridine-5'-monophosphate, resulting in the formation of thymidine 5'-monophosphate and dihydrofolate. This is the only de novo source of dTMP (a precursor to Thymine) in humans. [1]
2-deoxyuridine-5'-monophosphate(dUMP) + 5, 10-methylenetetrahydrofolate(CH2H4F) ⇌ thymidine 5'-monophosphate(dTMP) + Dihydrofolate(H2F)
| |||||||||||
References
- ↑ Kholodar SA, Finer-Moore JS, Swiderek K, Arafet K, Moliner V, Stroud RM, Kohen A. Caught in Action: X-ray Structure of Thymidylate Synthase with Noncovalent Intermediate Analog. Biochemistry. 2021 Apr 8. doi: 10.1021/acs.biochem.1c00063. PMID:33829766 doi:http://dx.doi.org/10.1021/acs.biochem.1c00063
- ↑ Kaneda S, Nalbantoglu J, Takeishi K, Shimizu K, Gotoh O, Seno T, Ayusawa D. Structural and functional analysis of the human thymidylate synthase gene. J Biol Chem. 1990 Nov 25;265(33):20277-84. PMID:2243092
- ↑ Rahman L, Voeller D, Rahman M, Lipkowitz S, Allegra C, Barrett JC, Kaye FJ, Zajac-Kaye M. Thymidylate synthase as an oncogene: a novel role for an essential DNA synthesis enzyme. Cancer Cell. 2004 Apr;5(4):341-51. PMID:15093541
- ↑ Finer-Moore JS, Fauman EB, Morse RJ, Santi DV, Stroud RM. Contribution of a salt bridge to binding affinity and dUMP orientation to catalytic rate: mutation of a substrate-binding arginine in thymidylate synthase. Protein Eng. 1996 Jan;9(1):69-75. PMID:9053905
Proteopedia Page Contributors and Editors (what is this?)
Michael O'Shaughnessy, Karsten Theis, Michal Harel, Alexander Berchansky, Shaylie Albright, Anna Postnikova, Kia Yang, Joel L. Sussman