User:George G. Papadeas/Sandbox VKOR
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
== Function: Method of Coagulation == | == Function: Method of Coagulation == | ||
=== Brief Overview === | === Brief Overview === | ||
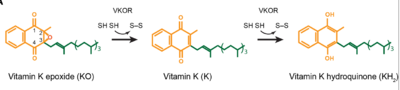
| - | The overall mechanism works to convert Vitamin K epoxide to an activated form of Vitamin K hydroquinone, as noted in Figure 1. The substrate will bind VKOR at the binding pocket in the <scene name='90/906893/Open_conformation/1'>open conformation</scene> and induce the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene>. Transition from open to closed conformation occurs with the oxidation of the C43-C51 disulfide bridge. Here, VKOR will utilize the second pair of catalytic cysteines, C132 and C135, to reduce KO into Vitamin K and Vitamin K into KH2. KH2 will be released from the binding fully activated and ready for use in the body. VKOR will reset, returning to the open conformation again, prepared for another substrate to bind. | + | The overall mechanism works to convert Vitamin K epoxide to an activated form of Vitamin K hydroquinone, as noted in Figure 1. The substrate will bind VKOR at the binding pocket in the <scene name='90/906893/Open_conformation/1'>open conformation</scene> and induce the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene>. Transition from open to closed conformation occurs with the oxidation of the C43-C51 disulfide bridge. Here, VKOR will utilize the second pair of <scene name='90/904314/Disulfide_bridge_stabilization/7'>catalytic cysteines</scene>, C132 and C135, to reduce KO into Vitamin K and Vitamin K into KH2. KH2 will be released from the binding fully activated and ready for use in the body. VKOR will reset, returning to the open conformation again, prepared for another substrate to bind. |
| - | === | + | |
| + | === Enzymatic Mechanism === | ||
[[Image:Catalytic Mech Pic.png |350 px| right| thumb]] | [[Image:Catalytic Mech Pic.png |350 px| right| thumb]] | ||
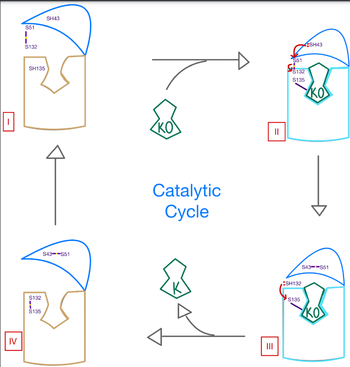
| - | The catalytic mechanism of VKOR is | + | The catalytic mechanism of VKOR is highly regulated and use <scene name='90/904314/Stage_4_catalytic_cycle/13'>four catalytic cysteine residues</scene> to activate Vitamin K necessary for blood coagulation. Figure 3 highlights these reactions that allow the substrate to be catalyzed to its active form through a series of 4 stages. The enzyme begins in <scene name='90/904314/Vkor_with_ko/1'>stage I</scene> in the open conformation with the cap domain open to allow substrate binding. Once a substrate binds, the cap domain transitions to the closed conformation when the C51-C132 disulfide bridge is attacked by reactive C43 located within the cap domain. This reaction forms a new disulfide bridge between C43 and C51 that pulls the cap domain over the binding pocket with the substrate bound to stabilize the closed conformation of VKOR. VKOR is now in <scene name='90/904314/Stage_2_catalytic_cycle/1'>stage II</scene>. Free cysteines are now available that provide strong stabilization of the closed conformation through interactions with the cap domain and the bound substrate. This puts the enzyme in <scene name='90/904314/Stage_3_catalytic_cycle/7'>stage III</scene>, where a free C135 is purposed to interact with the substrate within the binding pocket to stabilize it during activation. The catalytic free C132 located between the cap domain and helical tunnel is very reactive and will attack this C135 to break that interaction with the substrate and release the activated Vitamin K product into the blood stream to promote coagulation. Two very stable disulfide bridges between C43-C41 and C132-C135 are now present and VKOR is unbound, so the enzyme is in its final, unreactive <scene name='90/904314/Stage_4_catalytic_cycle/15'>stage IV</scene>. VKOR must undergo conformational changes to return to Stage 1 and reactivate its catalytic cysteines so that another molecule of Vitamin K can bind and be activated. |
== Disease and Treatment == | == Disease and Treatment == | ||
Revision as of 02:36, 18 April 2022
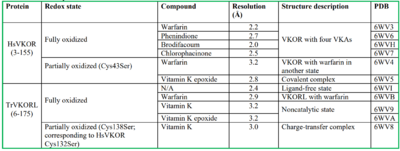
VKOR
| |||||||||||
References
1. DJin, Da-Yun, Tie, Jian-Ke, and Stafford, Darrel W. "The Conversion of Vitamin K Epoxide to Vitamin K Quinone and Vitamin K Quinone to Vitamin K Hydroquinone Uses the Same Active Site Cysteines." Biochemistry 2007 46 (24), 7279-7283 [1].
2. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. doi:10.1038/nature08720.
3. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: PMC7946407.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Unknown PubmedID 10.1126
- ↑ Unknown PubmedID 10.1021
- ↑ Unknown PubmedID 10.1126