We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1719
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
[[Image:Structure_overview_of_Mx_with_membrane.PNG|500px|right|thumb|'''Figure 1.''' Location of MRGPRX2 in plasma membrane.]] | [[Image:Structure_overview_of_Mx_with_membrane.PNG|500px|right|thumb|'''Figure 1.''' Location of MRGPRX2 in plasma membrane.]] | ||
= Introduction = | = Introduction = | ||
| - | [https://proteopedia.org/wiki/index.php/G_protein-coupled_receptors G protein-coupled receptors] (GPCRs) are the largest class of integral membrane proteins divided into five families; the [https://proteopedia.org/wiki/index.php/Sandbox_Reserved_895 rhodopsin family (class A)], the [https://proteopedia.org/wiki/index.php/4ers secretin family (class B)], the [https://proteopedia.org/wiki/index.php/6wiv glutamate family (class C)], the [https://proteopedia.org/wiki/index.php/6bd4 frizzled/taste family (class F)], and the [https://en.wikipedia.org/wiki/Adhesion_G_protein-coupled_receptor adhesion family]. | + | [https://proteopedia.org/wiki/index.php/G_protein-coupled_receptors G protein-coupled receptors] (GPCRs) are the largest class of integral membrane proteins divided into five families; the [https://proteopedia.org/wiki/index.php/Sandbox_Reserved_895 rhodopsin family (class A)], the [https://proteopedia.org/wiki/index.php/4ers secretin family (class B)], the [https://proteopedia.org/wiki/index.php/6wiv glutamate family (class C)], the [https://proteopedia.org/wiki/index.php/6bd4 frizzled/taste family (class F)], and the [https://en.wikipedia.org/wiki/Adhesion_G_protein-coupled_receptor adhesion family].<ref name= "Zhang 2006"/><ref name="Zhang 2015">DOI 10.14348/molcells.2015.0263</ref> GPCRs promote signal transduction activated by a variety of stimuli by undergoing a transmembrane domain conformational change once ligand binding to the N-terminus occurs. This allows for the transduction of a signal to a coupled, heterotrimeric G protein, which then dictates whether an intracellular signaling pathway will be initiated or inhibited. <ref name= "Zhang 2015"/><ref name= "Zhang 2006">DOI 10.1371/journal.pcbi.0020013</ref> |
Human Itch G-coupled protein receptors (GPCRs), or Mast cell-related GPCRs (MRGPRX), have been identified as pruritogenic receptors and are found in human sensory neurons, specifically in the connective tissue mast cells and dorsal root ganglia in humans.<ref name= "davidson2011">DOI: 10.1016/j.tins.2010.09.002</ref> They are classified as class A GPCRs, however, MRGPRX receptors respond to a diverse number of agonists, antagonists, and inverse agonists some of which are not typical ligands of class A receptors. MRGPRX receptors are involved in host defense, pseudo-allergic reactions, non-histaminergic itch, periodontitis, neurogenic inflammation, and inflammatory pain.<ref name= "davidson2011"/> | Human Itch G-coupled protein receptors (GPCRs), or Mast cell-related GPCRs (MRGPRX), have been identified as pruritogenic receptors and are found in human sensory neurons, specifically in the connective tissue mast cells and dorsal root ganglia in humans.<ref name= "davidson2011">DOI: 10.1016/j.tins.2010.09.002</ref> They are classified as class A GPCRs, however, MRGPRX receptors respond to a diverse number of agonists, antagonists, and inverse agonists some of which are not typical ligands of class A receptors. MRGPRX receptors are involved in host defense, pseudo-allergic reactions, non-histaminergic itch, periodontitis, neurogenic inflammation, and inflammatory pain.<ref name= "davidson2011"/> | ||
| Line 40: | Line 40: | ||
<scene name='90/904324/Active_site_residues/8'>Sub-pocket 2</scene> is formed by TM1, TM2, TM6, and TM7.<ref name="Can"/> This binding sub-pocket is much broader and allows for the binding of larger structures ('''Figure 3B''').<ref name="Can"/> The key residues involved are Trp243 and Phe170 that contributes to the high hydrophobicity of the binding pocket.<ref name="Can"/> The hydrophobicity of this binding pocket accounts for the large electrostatic difference observed between the two sub pockets demonstrated in '''Figure 2'''. | <scene name='90/904324/Active_site_residues/8'>Sub-pocket 2</scene> is formed by TM1, TM2, TM6, and TM7.<ref name="Can"/> This binding sub-pocket is much broader and allows for the binding of larger structures ('''Figure 3B''').<ref name="Can"/> The key residues involved are Trp243 and Phe170 that contributes to the high hydrophobicity of the binding pocket.<ref name="Can"/> The hydrophobicity of this binding pocket accounts for the large electrostatic difference observed between the two sub pockets demonstrated in '''Figure 2'''. | ||
| - | [[Image:Screen Shot 2022-04-18 at 1.35.31 PM.png|500px|center|thumb|'''Figure 3.''' (A). Cross-sectional views of electrostatic surface of MRGPRX2 sub-pocket 1 interaction with lysine 3 and (B) sub-pocket 2 interaction with phenylalanine 6 of cortistatin-14.]] | + | [[Image:Screen Shot 2022-04-18 at 1.35.31 PM.png|500px|center|thumb|'''Figure 3.''' (A). Cross-sectional views of electrostatic surface of MRGPRX2 sub-pocket 1 interaction with lysine 3 and (B). sub-pocket 2 interaction with phenylalanine 6 of cortistatin-14.]] |
== Ligand interactions == | == Ligand interactions == | ||
| Line 47: | Line 47: | ||
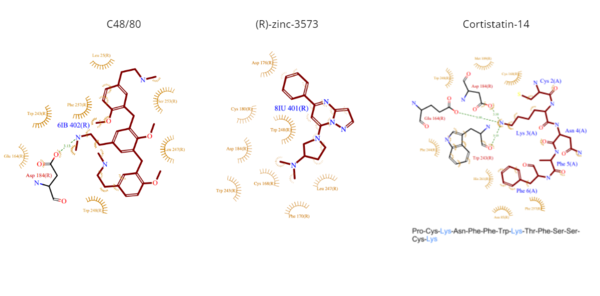
*<scene name='90/904324/Rzinc3573/7'>(R)-zinc-3573</scene> | *<scene name='90/904324/Rzinc3573/7'>(R)-zinc-3573</scene> | ||
| - | **(R)-zinc-3573 is an agonist that binds to MRGPRX2 when it is associated with a G<sub>i</sub> or G<sub>q</sub> protein.<ref name="Can">DOI: 10.1038/s41586-021-04126-6</ref> This agonist is a small cationic molecule that forms largely ionic interactions with the negatively-charged sub-pocket 1 and has no interactions with sub-pocket 2.<ref name="Can"/> (R)-zinc-3573 forms hydrogen bonds and hydrophobic interactions with Asp184 and Glu164 of sub-pocket 1.<ref name="Can"/> <ref>DOI: 10.1038/nchembio.2334</ref> | + | **(R)-zinc-3573 is an agonist that binds to MRGPRX2 when it is associated with a G<sub>i</sub> or G<sub>q</sub> protein.<ref name="Can">DOI: 10.1038/s41586-021-04126-6</ref> This agonist is a small cationic molecule that forms largely ionic interactions with the negatively-charged sub-pocket 1 and has no interactions with sub-pocket 2.<ref name="Can"/> (R)-zinc-3573 forms hydrogen bonds and hydrophobic interactions with Asp184 and Glu164 of sub-pocket 1.<ref name="Can"/><ref>DOI: 10.1038/nchembio.2334</ref> |
*<scene name='90/904324/Cortistatin-14/6'>Cortistatin-14</scene> | *<scene name='90/904324/Cortistatin-14/6'>Cortistatin-14</scene> | ||
| Line 65: | Line 65: | ||
=== Toggle switch === | === Toggle switch === | ||
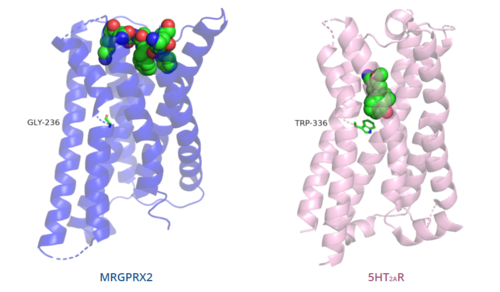
| - | The conserved <scene name='90/904324/5ht2a_toggle_switch/3'>toggle switch</scene> of class A GPCRs acts to activate or inhibit the transduction of the signaling cascade. Typical class A GPCRs contain the conserved ‘toggle switch’ Trp336. In MRGPRX2, this residue is replaced by <scene name='90/904324/Toggle_switch/7'>Gly236</scene>. <ref name="Can"/> By substituting the larger Trp residue with a smaller Gly residue, TM6 is shifted closer to TM3 on the extracellular side of the membrane and contributes to the more tightly packed and shallow binding pocket as compared to other canonical structures. The occluded binding pocket contributes the surface level binding depicted in '''Figure 5''', and allows for a greater variety of ligands interactions with MRGPRX2 as compared to other class A GPCRs, such as [https://proteopedia.org/wiki/index.php/5-hydroxytryptamine_receptor 5-HT<sub>2A</sub>R], [https://proteopedia.org/wiki/index.php/Adrenergic_receptor A<sub>2A</sub>R], and [https://proteopedia.org/wiki/index.php/Beta-2_Adrenergic_Receptor β<sub>2</sub>AR].<ref name="Can"/> | + | The conserved <scene name='90/904324/5ht2a_toggle_switch/3'>toggle switch</scene> of class A GPCRs acts to activate or inhibit the transduction of the signaling cascade. Typical class A GPCRs contain the conserved ‘toggle switch’ Trp336. In MRGPRX2, this residue is replaced by <scene name='90/904324/Toggle_switch/7'>Gly236</scene>.<ref name="Can"/> By substituting the larger Trp residue with a smaller Gly residue, TM6 is shifted closer to TM3 on the extracellular side of the membrane and contributes to the more tightly packed and shallow binding pocket as compared to other canonical structures. The occluded binding pocket contributes the surface level binding depicted in '''Figure 5''', and allows for a greater variety of ligands interactions with MRGPRX2 as compared to other class A GPCRs, such as [https://proteopedia.org/wiki/index.php/5-hydroxytryptamine_receptor 5-HT<sub>2A</sub>R], [https://proteopedia.org/wiki/index.php/Adrenergic_receptor A<sub>2A</sub>R], and [https://proteopedia.org/wiki/index.php/Beta-2_Adrenergic_Receptor β<sub>2</sub>AR].<ref name="Can"/> |
=== PIF/LLF motif === | === PIF/LLF motif === | ||
| - | The majority of class A GPCRs contain a conserved <scene name='90/904324/5ht2a/3'>PIF motif</scene> at the TM3-TM6 interface. <ref name="Can"/> | + | The majority of class A GPCRs contain a conserved <scene name='90/904324/5ht2a/3'>PIF motif</scene> at the TM3-TM6 interface.<ref name="Can"/> |
Canonically, the conserved PIF motif consists of a Pro, Ile, and Phe that transduce the signal produce by ligand binding through the TMD within conserved distances.<ref name="Can"/><ref>DOI: 10.1038/s41467-017-02257-x</ref> | Canonically, the conserved PIF motif consists of a Pro, Ile, and Phe that transduce the signal produce by ligand binding through the TMD within conserved distances.<ref name="Can"/><ref>DOI: 10.1038/s41467-017-02257-x</ref> | ||
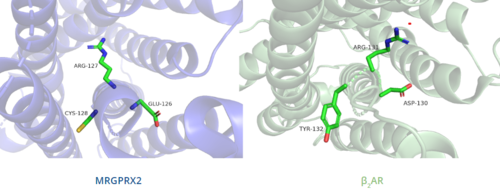
| - | In MRGPRX2, the PIF motif is changed to a <scene name='90/904324/Pifllf_motif/5'>LLF motif</scene>, in which the residues are not conserved at specific positions in the amino acid sequence, but instead are conserved at distances that allow them to interact.<ref name="Can"/> The residues that make up TM5 have shifted down two residues making Leu194 analogous to the position of the Pro in other GPCRs. Compared with other structures, such as [https://proteopedia.org/wiki/index.php/5-hydroxytryptamine_receptor 5-HT<sub>2A</sub>R], [https://proteopedia.org/wiki/index.php/Adrenergic_receptor A<sub>2A</sub>R], and [https://proteopedia.org/wiki/index.php/Beta-2_Adrenergic_Receptor β<sub>2</sub>AR], the TM6 helix of MRGPRX2 is closer to the TM3 helix due to the shift in residues, which accounts for the tighter packing of the transmembrane domain helices <ref name="Can"/>, thereby contributing to surface level ligand interactions. | + | In MRGPRX2, the PIF motif is changed to a <scene name='90/904324/Pifllf_motif/5'>LLF motif</scene>, in which the residues are not conserved at specific positions in the amino acid sequence, but instead are conserved at distances that allow them to interact.<ref name="Can"/> The residues that make up TM5 have shifted down two residues making Leu194 analogous to the position of the Pro in other GPCRs. Compared with other structures, such as [https://proteopedia.org/wiki/index.php/5-hydroxytryptamine_receptor 5-HT<sub>2A</sub>R], [https://proteopedia.org/wiki/index.php/Adrenergic_receptor A<sub>2A</sub>R], and [https://proteopedia.org/wiki/index.php/Beta-2_Adrenergic_Receptor β<sub>2</sub>AR], the TM6 helix of MRGPRX2 is closer to the TM3 helix due to the shift in residues, which accounts for the tighter packing of the transmembrane domain helices<ref name="Can"/>, thereby contributing to surface level ligand interactions. |
=== DRY/ERC motif === | === DRY/ERC motif === | ||
| Line 97: | Line 97: | ||
| - | Binding to the extracellular N-terminus domain triggers a transmembrane conformation change of MRGPRX2, which demonstrates a less significant change when compared to other class A GPCRs due to the surface level binding of the ligand to MRGPRX2.<ref name="Can"/> Once ligand binding and the conformational change to the active state have taken place, the signal is relayed to the α-subunit of the heterotrimeric G-protein.<ref name="nelson"/> The α-subunit will then exchange a GDP for GTP to initiate the dissociation of the α, β, and γ subunits.<ref name="nelson"/> During this dissociation, the α-subunit is able to travel away from the receptor in the plane of the membrane to bind to downstream effectors to produce a cellular response.<ref name="nelson"/> | ||
| + | Binding to the extracellular N-terminus domain triggers a transmembrane conformation change of MRGPRX2, which demonstrates a less significant change when compared to other class A GPCRs due to the surface level binding of the ligand to MRGPRX2.<ref name="Can"/> Once ligand binding and the conformational change to the active state have taken place, the signal is relayed to the α-subunit of the heterotrimeric G-protein.<ref name="nelson"/> The α-subunit will then exchange a GDP for GTP to initiate the dissociation of the α, β, and γ subunits.<ref name="nelson"/> During this dissociation, the α-subunit is able to travel away from the receptor in the plane of the membrane to bind to downstream effectors to produce a cellular response.<ref name="nelson"/> | ||
Revision as of 18:49, 18 April 2022
Human Itch G-Coupled Protein Receptors
| |||||||||||