Sandbox Reserved 1723
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== G-Protein Coupled Receptors == | == G-Protein Coupled Receptors == | ||
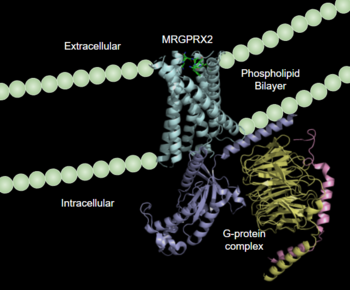
| - | [https://proteopedia.org/wiki/index.php/G_protein-coupled_receptors G-protein coupled receptors](GCPRs) are a large family of cell surface membrane receptors. Once bound to a wide variety of extracellular ligands, GCPRs undergo a conformational change and relay information to intracellular secondary messengers <ref name="Thal">Thal, David M., et al. "Structural insights into G-protein-coupled receptor allostery." Nature, Nature Publishing Group, 04 July 2018, https://www.nature.com/articles/s41586-018-0259-z</ref>. This G protein activation results in a cellular response dependent on the ligand bound and location of the GPCR in the body. GCPRs can be broken down into five families: the rhodopsin family (class A), the secretin family (class B), the adhesion family, the glutamate family (class C), and the frizzled/taste family (class F) <ref name="Zhang">PMID: 26467290</ref>. All of the families have a similar transmembrane (TM) domain consisting of seven <scene name='90/904328/7tm_domain_pt_4/1'>α-helices</scene> complexed with intracellular G proteins. | + | [https://proteopedia.org/wiki/index.php/G_protein-coupled_receptors G-protein coupled receptors](GCPRs) are a large family of cell surface membrane receptors. Once bound to a wide variety of extracellular ligands, GCPRs undergo a conformational change and relay information to intracellular secondary messengers <ref name="Thal">Thal, David M., et al. "Structural insights into G-protein-coupled receptor allostery." Nature, Nature Publishing Group, 04 July 2018, https://www.nature.com/articles/s41586-018-0259-z</ref>. This G protein activation results in a cellular response dependent on the ligand bound and location of the GPCR in the body. GCPRs can be broken down into five families: the rhodopsin family (class A), the secretin family (class B), the adhesion family, the glutamate family (class C), and the frizzled/taste family (class F) <ref name="Zhang">PMID: 26467290</ref>. All of the families have a similar transmembrane (TM) domain consisting of seven <scene name='90/904328/7tm_domain_pt_4/1'>α-helices</scene> <scene name='90/904328/7tm_domain_pt_3/3'>α-helices</scene>complexed with intracellular G proteins. |
=== Class A GCPRs === | === Class A GCPRs === | ||
Revision as of 18:11, 18 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
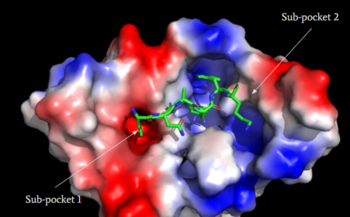
Human Itch GPCR
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Cao, Can, et al. "Structure, function and pharmacology of human itch GPCRs." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04126-6
- ↑ Thal, David M., et al. "Structural insights into G-protein-coupled receptor allostery." Nature, Nature Publishing Group, 04 July 2018, https://www.nature.com/articles/s41586-018-0259-z
- ↑ 3.0 3.1 Zhang D, Zhao Q, Wu B. Structural Studies of G Protein-Coupled Receptors. Mol Cells. 2015 Oct;38(10):836-42. doi: 10.14348/molcells.2015.0263. Epub 2015, Oct 15. PMID:26467290 doi:http://dx.doi.org/10.14348/molcells.2015.0263
- ↑ 4.0 4.1 4.2 Zhou Q, Yang D, Wu M, Guo Y, Guo W, Zhong L, Cai X, Dai A, Jang W, Shakhnovich EI, Liu ZJ, Stevens RC, Lambert NA, Babu MM, Wang MW, Zhao S. Common activation mechanism of class A GPCRs. Elife. 2019 Dec 19;8. pii: 50279. doi: 10.7554/eLife.50279. PMID:31855179 doi:http://dx.doi.org/10.7554/eLife.50279
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Yang, Fan, et al. "Structure, function and pharmacology of human itch receptor complexes." Nature, Nature Publishing Group, 17 November 2021, https://www.nature.com/articles/s41586-021-04077-y
- ↑ 6.0 6.1 Schonegge, Anne-Marie, et al. "Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity." Nature, Nature Publishing Group, 18 December 2017, https://www.nature.com/articles/s41467-017-02257-x
- ↑ Sandoval, A., et al. "The Molecular Switching Mechanism at the Conserved D(E)RY Motif in Class-A GPCRs." Biophysical journal, 111(1), 79-89. https://doi.org/10.1016/j.bpj.2016.06.004
- ↑ Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 May;39(5):233-44. doi: 10.1016/j.tibs.2014.03.002. Epub , 2014 Apr 21. PMID:24767681 doi:http://dx.doi.org/10.1016/j.tibs.2014.03.002
- ↑ Babina, M., et al. "MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway." Journal of Investigative Dermatology, 141(6), 1286-1296. https://doi.org/10.1016/j.jid.2020.09.017