We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1724
From Proteopedia
(Difference between revisions)
| Line 24: | Line 24: | ||
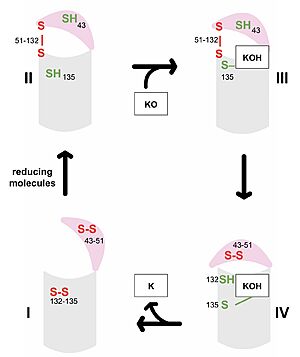
[[Image:VKOR_Catalytic_cycle.jpg|300 px|right|thumb|'''Figure 3. Catalytic Cycle of VKOR''' VKOR's luminal domain is represented by a the pink semicircle and the transmembrane domain is represented by the gray cylinder. Step I and II represent open conformations of VKOR and steps III and IV represent closed conformations.]] | [[Image:VKOR_Catalytic_cycle.jpg|300 px|right|thumb|'''Figure 3. Catalytic Cycle of VKOR''' VKOR's luminal domain is represented by a the pink semicircle and the transmembrane domain is represented by the gray cylinder. Step I and II represent open conformations of VKOR and steps III and IV represent closed conformations.]] | ||
| - | The first step <scene name='90/904329/Cat_cycle_i/7'>(step I)</scene> of the catalytic cycle (Figure 3) is the wild type open conformation. This step is characterized by an open cap domain with disulfide bonds between cysteines 43 and 51 and between cysteines 132 and 135 <ref name="Liu">PMID:33154105</ref>. The second step <scene name='90/904329/Cat_cycle_2/8'>(step II)</scene> of the catalytic cycle is a partially oxidized open conformation. This step is characterized by a disulfide bond between the luminal and transmembrane domain (Fig 3, step II). The transmembrane domain contains a free Cys135 and the luminal domain contains a free Cys43 <ref name="Liu">PMID:33154105</ref>. Step II is labeled as open because no ligand exists within its binding pocket despite the disulfide bridge that connects the luminal and transmembrane domains. The next step of the cycle <scene name='90/904329/Cat_cycle_3/8'>(step III)</scene> is a closed structure with an intact disulfide bond between Cys51 and Cys132. Cys135 is not involved in a disulfide bridge and | + | The first step <scene name='90/904329/Cat_cycle_i/7'>(step I)</scene> of the catalytic cycle (Figure 3) is the wild type open conformation. This step is characterized by an open cap domain with disulfide bonds between cysteines 43 and 51 and between cysteines 132 and 135 <ref name="Liu">PMID:33154105</ref>. The second step <scene name='90/904329/Cat_cycle_2/8'>(step II)</scene> of the catalytic cycle is a partially oxidized open conformation. This step is characterized by a disulfide bond between the luminal and transmembrane domain (Fig 3, step II). The transmembrane domain contains a free Cys135 and the luminal domain contains a free Cys43 <ref name="Liu">PMID:33154105</ref>. Step II is labeled as open because no ligand exists within its binding pocket despite the disulfide bridge that connects the luminal and transmembrane domains. The next step of the cycle <scene name='90/904329/Cat_cycle_3/8'>(step III)</scene> is a closed structure with an intact disulfide bond between Cys51 and Cys132. Cys135 is not involved in a disulfide bridge and instead reacts with substrate by forming a stable adduct with KOH or K. This binding induces the closed conformation and uses Cys43 in the luminal membrane for electron transfer <ref name="Liu">PMID:33154105</ref>. The final step <scene name='90/904329/Cat_cycle_4/4'>(step IV)</scene> of the catalytic cycle is the last closed conformation. The Cys51-Cys132 bond is broken as Cys43 bonds with Cys51, recreating the disulfide bridge pattern of the open state. Cys132 is then free to bond with Cys135, releasing the product that was bound to the Cys135. Overall the catalytic cycle of VKOR is dependent on open and closed conformational changes of the protein and ultimately is used to generate vitamin K from vitamin K epoxide <ref name="Liu">PMID:33154105</ref>. |
== Medical Relevance == | == Medical Relevance == | ||
Current revision
–
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
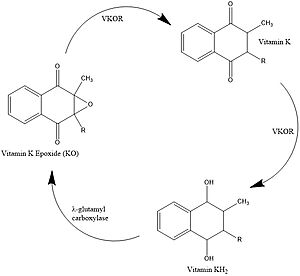
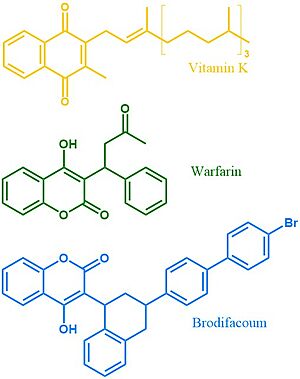
Vitamin K Epoxide Reductase
| |||||||||||
References
- ↑ 1.0 1.1 Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005 Aug;3(8):1873-8. doi: 10.1111/j.1538-7836.2005.01419.x. PMID:16102054 doi:http://dx.doi.org/10.1111/j.1538-7836.2005.01419.x
- ↑ 2.0 2.1 Blanchard RA, Furie BC, Jorgensen M, Kruger SF, Furie B. Acquired vitamin K-dependent carboxylation deficiency in liver disease. N Engl J Med. 1981 Jul 30;305(5):242-8. doi: 10.1056/NEJM198107303050502. PMID:6165889 doi:http://dx.doi.org/10.1056/NEJM198107303050502
- ↑ Swanson JC, Suttie JW. Vitamin K dependent in vitro production of prothrombin. Biochemistry. 1982 Nov 9;21(23):6011-8. doi: 10.1021/bi00266a044. PMID:6758841 doi:http://dx.doi.org/10.1021/bi00266a044
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667
- ↑ 5.0 5.1 Patel S, Singh R, Preuss CV, Patel N. Warfarin PMID:29261922
- ↑ Wu S, Chen X, Jin DY, Stafford DW, Pedersen LG, Tie JK. Warfarin and vitamin K epoxide reductase: a molecular accounting for observed inhibition. Blood. 2018 Aug 9;132(6):647-657. doi: 10.1182/blood-2018-01-830901. Epub 2018, May 9. PMID:29743176 doi:http://dx.doi.org/10.1182/blood-2018-01-830901
- ↑ 7.0 7.1 Chong YK, Mak TW. Superwarfarin (Long-Acting Anticoagulant Rodenticides) Poisoning: from Pathophysiology to Laboratory-Guided Clinical Management. Clin Biochem Rev. 2019 Nov;40(4):175-185. doi: 10.33176/AACB-19-00029. PMID:31857739 doi:http://dx.doi.org/10.33176/AACB-19-00029
Student Contributors
Izabella Jordan, Emma Varness