This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:George G. Papadeas/Sandbox VKOR
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
== Structural Highlights== | == Structural Highlights== | ||
VKOR has many key structural components that allow it to maintain proper functionality and catalytic abilities. The main part of the enzyme that contains the active site is a <scene name='90/904314/Stage_4_catalytic_cycle/11'>4 helix bundle</scene> binding pocket where main catalytic activity occurs. The VKOR binding pocket provides specific substrate binding via highly conserved residues that recognize the target substrates. The pocket works in conjunction with the cap domain. The cap domain is a helical component of VKOR that facilitates conformational transitions from the <scene name='90/906893/Open_conformation/1'>open conformation</scene> to the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene> once a substrate binds. Interactions between the cap domain, binding pocket, and the bound protein are critical to achieve full activation of Vitamin K. Another necessary part of the structure is the anchor. The anchor serves as a way to hold VKOR in the proper orientation within the cell membrane such that all enzymatic components are in the correct proximity for substrate binding and catalysis. Vital to the VKOR structure and these components are two disulfide bridges. The first appears slightly above the binding pocket between C132 and C135. The second occurs within the cap domain between C43 and C51. These cysteines are catalytic residues that also aid in the transition of VKOR from the open conformation to the closed conformation and the reduction of KO. | VKOR has many key structural components that allow it to maintain proper functionality and catalytic abilities. The main part of the enzyme that contains the active site is a <scene name='90/904314/Stage_4_catalytic_cycle/11'>4 helix bundle</scene> binding pocket where main catalytic activity occurs. The VKOR binding pocket provides specific substrate binding via highly conserved residues that recognize the target substrates. The pocket works in conjunction with the cap domain. The cap domain is a helical component of VKOR that facilitates conformational transitions from the <scene name='90/906893/Open_conformation/1'>open conformation</scene> to the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene> once a substrate binds. Interactions between the cap domain, binding pocket, and the bound protein are critical to achieve full activation of Vitamin K. Another necessary part of the structure is the anchor. The anchor serves as a way to hold VKOR in the proper orientation within the cell membrane such that all enzymatic components are in the correct proximity for substrate binding and catalysis. Vital to the VKOR structure and these components are two disulfide bridges. The first appears slightly above the binding pocket between C132 and C135. The second occurs within the cap domain between C43 and C51. These cysteines are catalytic residues that also aid in the transition of VKOR from the open conformation to the closed conformation and the reduction of KO. | ||
| + | |||
| + | === Cap Domain === | ||
| + | === Anchor === | ||
=== Active Site === | === Active Site === | ||
Within the four transmembrane helices lies the <scene name='90/906893/Binding_pocket/1'>binding pocket</scene>. The binding pocket is comprised of a <scene name='90/906893/Hydrophobic/2'>hydrophobic region</scene> containing <scene name='90/906893/Active_site/7'>two hydrophilic residues</scene>, N80 and Y139, that interact with substrates. The hydrophobic pocket provides specificity to the region while the hydrophilic residues hydrogen bond to the substrate, providing recognition and increasing specificity. The <scene name='90/906893/Disulfide_-_132/1'>C132-C135 disulfide bridge</scene> above the binding pocket provides stabilization when a substrate is bound. This bridge provides increased stability for the binding site as it interacts with and binds substrates or inhibitors. The hydrophilic residues provide <scene name='90/906893/K_hbonds/1'>hydrogen bonds</scene> when interacting with substrates for specificity and recognition. Upon binding, VKOR will transition into the closed conformation allowing the catalytic mechanism to commence. | Within the four transmembrane helices lies the <scene name='90/906893/Binding_pocket/1'>binding pocket</scene>. The binding pocket is comprised of a <scene name='90/906893/Hydrophobic/2'>hydrophobic region</scene> containing <scene name='90/906893/Active_site/7'>two hydrophilic residues</scene>, N80 and Y139, that interact with substrates. The hydrophobic pocket provides specificity to the region while the hydrophilic residues hydrogen bond to the substrate, providing recognition and increasing specificity. The <scene name='90/906893/Disulfide_-_132/1'>C132-C135 disulfide bridge</scene> above the binding pocket provides stabilization when a substrate is bound. This bridge provides increased stability for the binding site as it interacts with and binds substrates or inhibitors. The hydrophilic residues provide <scene name='90/906893/K_hbonds/1'>hydrogen bonds</scene> when interacting with substrates for specificity and recognition. Upon binding, VKOR will transition into the closed conformation allowing the catalytic mechanism to commence. | ||
| - | == | + | ==Catalytic Mechanism of VKOR== |
| - | + | ||
| - | + | ||
| - | + | ||
=== Brief Overview === | === Brief Overview === | ||
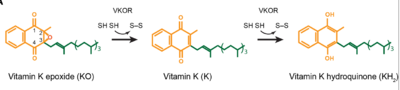
The overall mechanism works to convert Vitamin K epoxide to an activated form of Vitamin K hydroquinone, as noted in Figure 1. The substrate will bind VKOR at the binding pocket in the <scene name='90/906893/Open_conformation/1'>open conformation</scene> and induce the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene>. Transition from open to closed conformation occurs with the oxidation of the C43-C51 disulfide bridge. Here, VKOR will utilize the second pair of <scene name='90/904314/Disulfide_bridge_stabilization/7'>catalytic cysteines</scene>, C132 and C135, to reduce KO into Vitamin K and Vitamin K into KH2. KH2 will be released from the binding fully activated and ready for use in the body. VKOR will reset, returning to the open conformation again, prepared for another substrate to bind. | The overall mechanism works to convert Vitamin K epoxide to an activated form of Vitamin K hydroquinone, as noted in Figure 1. The substrate will bind VKOR at the binding pocket in the <scene name='90/906893/Open_conformation/1'>open conformation</scene> and induce the <scene name='90/906893/Closed_conformation/4'>closed conformation</scene>. Transition from open to closed conformation occurs with the oxidation of the C43-C51 disulfide bridge. Here, VKOR will utilize the second pair of <scene name='90/904314/Disulfide_bridge_stabilization/7'>catalytic cysteines</scene>, C132 and C135, to reduce KO into Vitamin K and Vitamin K into KH2. KH2 will be released from the binding fully activated and ready for use in the body. VKOR will reset, returning to the open conformation again, prepared for another substrate to bind. | ||
| - | |||
=== Enzymatic Mechanism === | === Enzymatic Mechanism === | ||
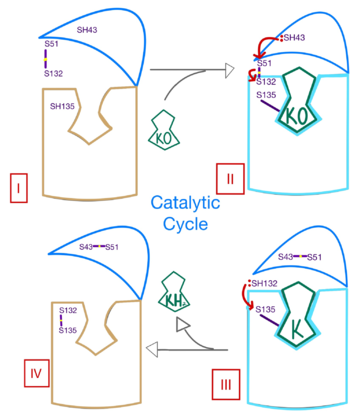
| - | [[Image: | + | [[Image:Ss_of_catalytic_mech.png|350 px| right| thumb | Figure 3. Mechanism of VKOR.]] |
| - | The catalytic mechanism of VKOR is highly regulated and use <scene name='90/904314/Stage_4_catalytic_cycle/ | + | The catalytic mechanism of VKOR is highly regulated and use <scene name='90/904314/Stage_4_catalytic_cycle/17'>four catalytic cysteine residues</scene> to activate Vitamin K necessary for blood coagulation. Figure 3 highlights these reactions that allow the substrate to be catalyzed to its active form through a series of 4 stages. The enzyme begins in <scene name='90/904314/Vkor_with_ko/1'>stage I</scene> in the open conformation with the cap domain open to allow substrate binding. Once a substrate binds, the cap domain transitions to the closed conformation, initiating <scene name='90/904314/Stage_2_catalytic_cycle/2'>stage II</scene> when the C51-C132 disulfide bridge is attacked by reactive C43 located within the cap domain. This reaction forms a new disulfide bridge between C43 and C51 that pulls the cap domain over the binding pocket with the substrate bound to stabilize the closed conformation of VKOR. VKOR is now in stage II. Free cysteines are now available that provide strong stabilization of the closed conformation through interactions with the cap domain and the bound substrate. This puts the enzyme in <scene name='90/904314/Stage_3_catalytic_cycle/8'>stage III</scene>, where a free C135 is purposed to interact with the substrate within the binding pocket to stabilize it during activation. The catalytic free C132 located between the cap domain and helical tunnel is very reactive and will attack this C135 to break that interaction with the substrate and release the activated Vitamin K product into the blood stream to promote coagulation. Two very stable disulfide bridges between C43-C41 and C132-C135 are now present and VKOR is unbound, so the enzyme is in its final, unreactive <scene name='90/904314/Stage_4_catalytic_cycle/15'>stage IV</scene>. VKOR must undergo conformational changes to return to Stage 1 and reactivate its catalytic cysteines so that another molecule of Vitamin K can bind and be activated. |
== Disease and Treatment == | == Disease and Treatment == | ||
=== Afflictions === | === Afflictions === | ||
| - | Since activated Vitamin K plays a crucial role in blood coagulation, defects in the function and enzymatic activity of VKOR may detrimentally effect on Vitamin K's ability to promote blood clotting. Mutations in VKOR also increase susceptibility to vascular diseases, such as a stroke | + | Since activated Vitamin K plays a crucial role in blood coagulation, defects in the function and enzymatic activity of VKOR may detrimentally effect on Vitamin K's ability to promote blood clotting. Mutations in VKOR also increase susceptibility to vascular diseases, such as a stroke <ref>DOI 10.1161/CIRCULATIONAHA.105.580167</ref>. Vitamin K is also important in maintaining bone health with inactivity of VKOR linked to decreased bone density and osteoporosis osteoporosis<ref>DOI 10.7759/cureus.10816</ref>. |
| + | |||
=== Inhibition === | === Inhibition === | ||
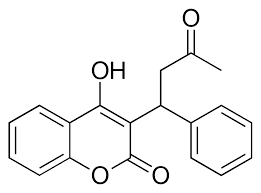
[[Image:Warfarin.png |400 px| right| thumb | Figure 4. Structure of Warfarin.]] | [[Image:Warfarin.png |400 px| right| thumb | Figure 4. Structure of Warfarin.]] | ||
| - | The most common way to treat blood clotting is using the VKOR inhibitor, <scene name='90/904314/Vkor_with_warfarin_bound/1'>warfarin</scene>. [https://en.wikipedia.org/wiki/Warfarin Warfarin] outcompetes KO, such that Vitamin K cannot be activated to promote coagulation in the blood. Warfarin will enter the binding pocket of VKOR, creating strong <scene name='90/904314/Vkor_with_warfarin_bound/2'>hydrogen bonds</scene> with the active site. Mutations of VKOR can lead to warfarin resistance which decreases its anticoagulation effects. Different mutations introduce varying degrees of resistance. These mutations are important to recognize as super-warfarin's can be overly effective in anticoagulation and become detrimental to blood flow. | + | The most common way to treat blood clotting is using the VKOR inhibitor, <scene name='90/904314/Vkor_with_warfarin_bound/1'>warfarin</scene>. [https://en.wikipedia.org/wiki/Warfarin Warfarin] outcompetes KO<ref>PMID: 29261922</ref>, such that Vitamin K cannot be activated to promote coagulation in the blood. Warfarin will enter the binding pocket of VKOR, creating strong <scene name='90/904314/Vkor_with_warfarin_bound/2'>hydrogen bonds</scene> with the active site residues, N80 and Y139. Mutations of VKOR can lead to warfarin resistance which decreases its anticoagulation effects. Different mutations introduce varying degrees of resistance. These mutations are important to recognize as [https://en.wikipedia.org/wiki/Superwarfarin super-warfarin's] can be overly effective in anticoagulation and become detrimental to blood flow. |
=== Mutations === | === Mutations === | ||
| - | + | Within the <scene name='90/904314/Active_site/2'>binding pocket</scene> mutations of the <scene name='90/904314/Vkor_with_warfarin_bound/4'>active site residues</scene> can occur. These mutations can be detrimental to the VKOR structure and function<ref>DOI 10.1126/science.abc5667</ref>. Two of the most common mutations occur to residues N80 and Y139 mutating them to <scene name='90/904314/Active_site_mutations/2'>A80 and F139</scene>. The change in polarity of these mutations from polar to nonpolar will cause a decrease in recognition and stabilization due to the inability to provide hydrogen bonds. | |
| - | + | ||
| - | + | ||
| - | + | ||
</StructureSection> | </StructureSection> | ||
| - | |||
== References == | == References == | ||
| - | 1. DJin, Da-Yun, Tie, Jian-Ke, and Stafford, Darrel W. "The Conversion of Vitamin K Epoxide to Vitamin K Quinone and Vitamin K Quinone to Vitamin K Hydroquinone Uses the Same Active Site Cysteines." Biochemistry 2007 46 (24), 7279-7283 [https://pubs.acs.org/doi/10.1021/bi700527j]. | ||
| - | |||
| - | 2. Li, Weikai et al. “Structure of a bacterial homologue of vitamin K epoxide reductase.” Nature vol. 463,7280 (2010): 507-12. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2919313/ doi:10.1038/nature08720]. | ||
| - | |||
| - | 3. Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2021 Jan 1;371(6524):eabc5667. doi: 10.1126/science.abc5667. Epub 2020 Nov 5. PMID: 33154105; PMCID: [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7946407/ PMC7946407]. | ||
| - | |||
<references/> | <references/> | ||
Revision as of 00:00, 19 April 2022
VKOR
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Unknown PubmedID 10.1126
- ↑ Unknown PubmedID 10.1021
- ↑ Unknown PubmedID 10.1126
- ↑ Unknown PubmedID 10.1126
- ↑ Wang Y, Zhang W, Zhang Y, Yang Y, Sun L, Hu S, Chen J, Zhang C, Zheng Y, Zhen Y, Sun K, Fu C, Yang T, Wang J, Sun J, Wu H, Glasgow WC, Hui R. VKORC1 haplotypes are associated with arterial vascular diseases (stroke, coronary heart disease, and aortic dissection). Circulation. 2006 Mar 28;113(12):1615-21. doi: 10.1161/CIRCULATIONAHA.105.580167., Epub 2006 Mar 20. PMID:16549638 doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.105.580167
- ↑ Elshaikh AO, Shah L, Joy Mathew C, Lee R, Jose MT, Cancarevic I. Influence of Vitamin K on Bone Mineral Density and Osteoporosis. Cureus. 2020 Oct 5;12(10):e10816. doi: 10.7759/cureus.10816. PMID:33173624 doi:http://dx.doi.org/10.7759/cureus.10816
- ↑ Patel S, Singh R, Preuss CV, Patel N. Warfarin PMID:29261922
- ↑ Liu S, Li S, Shen G, Sukumar N, Krezel AM, Li W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science. 2020 Nov 5. pii: science.abc5667. doi: 10.1126/science.abc5667. PMID:33154105 doi:http://dx.doi.org/10.1126/science.abc5667