We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1726
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='7N00' size='350' side='right' caption=' Structure of Anaplastic Lymphoma Kinase [https://www.rcsb.org/structure/7N00 7N00]' scene='90/904331/Alk_full/1'> | <StructureSection load='7N00' size='350' side='right' caption=' Structure of Anaplastic Lymphoma Kinase [https://www.rcsb.org/structure/7N00 7N00]' scene='90/904331/Alk_full/1'> | ||
== Background == | == Background == | ||
| - | Anaplastic Lymphoma Kinase (ALK) is a [https://en.wikipedia.org/wiki/Transmembrane_protein transmembrane] receptor and a member of the family of [https://proteopedia.org/wiki/index.php/Receptor_tyrosine_kinases Receptor Tyrosine Kinases (RTKs)]<ref name="Iwahara">PMID:9053841</ref>. RTKs are a family of biomolecules that are primarily responsible for biosignaling pathways such as the insulin signaling pathway. ALK was identified as a novel tyrosine phosphoprotein in 1994 in an analysis of [https://lymphoma.org/aboutlymphoma/nhl/alcl/ Anaplastic Large-Cell Lymphoma], the protein's namesake.<ref name ="Huang" /> A full analysis and characterization of ALK was completed in 1997, properly identifying it as a RTK, and linking it closely to [https://en.wikipedia.org/wiki/Leukocyte_receptor_tyrosine_kinase Leukocyte Tyrosine Kinase] (LTK).<ref name ="Huang" /> ALK's normal activity as a receptor tyrosine kinase is to transfer a gamma-phosphate group from adenosine triphosphate (ATP) to a tyrosine residue on it's substrate.<ref name ="Huang" /> ALK is one of more than 50 RTKs encoded within the human genome, <ref name ="Huang" /> and it's tyrosine kinase activity seems to be especially important in the developing nervous system. <ref name ="Huang" /> ALK is most commonly associated with oncogenesis, as various factors, including overstimulation, lead to extreme cell proliferation. | + | Anaplastic Lymphoma Kinase (ALK) is a [https://en.wikipedia.org/wiki/Transmembrane_protein transmembrane] receptor and a member of the family of [https://proteopedia.org/wiki/index.php/Receptor_tyrosine_kinases Receptor Tyrosine Kinases (RTKs)]<ref name="Iwahara">PMID:9053841</ref>. RTKs are a family of biomolecules that are primarily responsible for biosignaling pathways such as the insulin signaling pathway. ALK was identified as a novel tyrosine phosphoprotein in 1994 in an analysis of [https://lymphoma.org/aboutlymphoma/nhl/alcl/ Anaplastic Large-Cell Lymphoma], the protein's namesake.<ref name ="Huang" /> A full analysis and characterization of ALK was completed in 1997, properly identifying it as a RTK, and linking it closely to [https://en.wikipedia.org/wiki/Leukocyte_receptor_tyrosine_kinase Leukocyte Tyrosine Kinase] (LTK).<ref name ="Huang" /> ALK's normal activity as a receptor tyrosine kinase is to transfer a gamma-phosphate group from adenosine triphosphate (ATP) to a tyrosine residue on it's substrate.<ref name ="Huang" /> ALK is one of more than 50 RTKs encoded within the human genome, <ref name ="Huang" /> and it's tyrosine kinase activity seems to be especially important in the developing nervous system. <ref name ="Huang" /> ALK is most commonly associated with oncogenesis, as various factors, including overstimulation, lead to extreme cell proliferation. It is primarily found in the fetal and infant developing nervous system, however when associated with cancer it can be found in systems other than the nervous system. Examples of these include colon and prostate cancer, where ALK is not normally expressed. This particular protein is interesting in part due to its unique structure, of that it shares the most similarity with LTKs, and it's popular association with devastating cancers. |
== Structure == | == Structure == | ||
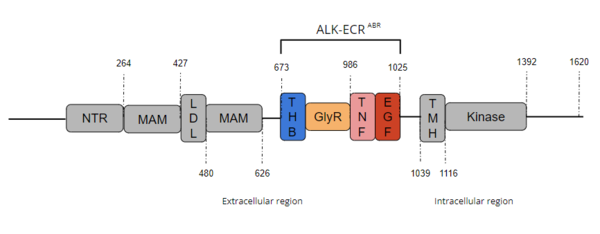
ALK is a close homolog of LTK, and together these two homologues constitute a subgroup within the superfamily of [https://proteopedia.org/wiki/index.php/Insulin_receptor insulin receptors]<ref name="Della Corte" />. ALK is composed of three primary regions: the extracellular region, the transmembrane region, and the intracellular region. [[Image:Full ALK Structure Graphic.PNG|600 px|right|thumb|Figure 1. Overview of Anaplastic Lymphoma Kinase Structure with domains where known structure are color coordinated and other domains are grayed out. The abbreviations are as follows: NTR-N-terminal Region, MAM-Meprin-A-5 protein-receptor protein tyrosine phosphatase μ, LDL-low-density lipoprotein receptor class A, THB-three helix bundle-like, GlyR-poly-glycine region, TNF-tumor necrosis factor-like domain, EGF-epidermal growth factor-like, TMH-transmembrane helix]] The extracellular region of ALK contains 8 total domains within 2 fragments. A Three Helix Bundle-like domain (THB-like), a Poly-Glycine domain (GlyR), a Tumor Necrosis Factor-like domain (TNF-like), and an Epidermal Growth Factor-like domain (EGF-like) make up the ligand binding fragment while a N-terminal domain, two [https://en.wikipedia.org/wiki/Meprin_A meprin–A-5] protein–receptor protein tyrosine phosphatase μ (MAM) domains and a [https://en.wikipedia.org/wiki/Low-density_lipoprotein low-density lipoprotein] receptor class A (LDL) domain sandwiched between the two MAM domains make up the second fragment. All four domains of the ligand binding fragment of the extracellular region contribute to ligand-binding <ref name ="Huang" />. The presence of an LDL domain sandwiched by two MAM domains is a unique feature that ALK does not share with other RTKs. The purpose behind this unique difference is still unclear, but the MAM region has been hypothesized to play a role in cell-cell signaling<ref name="Palmer">PMID:19459784</ref>. The [https://en.wikipedia.org/wiki/Transmembrane_domain transmembrane helical region] (TMH) bridges the gap between the intracellular and extracellular regions. The intracellular tyrosine kinase region features the Kinase domain and the C-terminal end (Figure 1). | ALK is a close homolog of LTK, and together these two homologues constitute a subgroup within the superfamily of [https://proteopedia.org/wiki/index.php/Insulin_receptor insulin receptors]<ref name="Della Corte" />. ALK is composed of three primary regions: the extracellular region, the transmembrane region, and the intracellular region. [[Image:Full ALK Structure Graphic.PNG|600 px|right|thumb|Figure 1. Overview of Anaplastic Lymphoma Kinase Structure with domains where known structure are color coordinated and other domains are grayed out. The abbreviations are as follows: NTR-N-terminal Region, MAM-Meprin-A-5 protein-receptor protein tyrosine phosphatase μ, LDL-low-density lipoprotein receptor class A, THB-three helix bundle-like, GlyR-poly-glycine region, TNF-tumor necrosis factor-like domain, EGF-epidermal growth factor-like, TMH-transmembrane helix]] The extracellular region of ALK contains 8 total domains within 2 fragments. A Three Helix Bundle-like domain (THB-like), a Poly-Glycine domain (GlyR), a Tumor Necrosis Factor-like domain (TNF-like), and an Epidermal Growth Factor-like domain (EGF-like) make up the ligand binding fragment while a N-terminal domain, two [https://en.wikipedia.org/wiki/Meprin_A meprin–A-5] protein–receptor protein tyrosine phosphatase μ (MAM) domains and a [https://en.wikipedia.org/wiki/Low-density_lipoprotein low-density lipoprotein] receptor class A (LDL) domain sandwiched between the two MAM domains make up the second fragment. All four domains of the ligand binding fragment of the extracellular region contribute to ligand-binding <ref name ="Huang" />. The presence of an LDL domain sandwiched by two MAM domains is a unique feature that ALK does not share with other RTKs. The purpose behind this unique difference is still unclear, but the MAM region has been hypothesized to play a role in cell-cell signaling<ref name="Palmer">PMID:19459784</ref>. The [https://en.wikipedia.org/wiki/Transmembrane_domain transmembrane helical region] (TMH) bridges the gap between the intracellular and extracellular regions. The intracellular tyrosine kinase region features the Kinase domain and the C-terminal end (Figure 1). | ||
| Line 17: | Line 17: | ||
''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ||
==== Epidermal Growth Factor-like Domain ==== | ==== Epidermal Growth Factor-like Domain ==== | ||
| - | Unlike the poly-Glycine helices, the <scene name='90/904331/Egf_like_domain/3'>Epidermal Growth Factor-like Domain</scene> is malleable and repositioning of this domain is essential for activation of the protein.<ref name="Reshetnyak" /> This domain undergoes conformational changes upon ligand binding and when in contact with the TNF-like domain.<ref name="Reshetnyak" /> The <scene name='90/904332/Tnf_egf_interface/3'>interface between the EGF-like and TNF-like domains</scene> consists of primarily hydrophobic residues, which enables their flexibility with regards to one another.<ref name="Reshetnyak" /> Major motifs in the EGF-like domain are major and minor β-hairpins, which are stabilized by 3 conserved disulfide bridges. <ref name="Reshetnyak" /> | + | Unlike the poly-Glycine helices, the <scene name='90/904331/Egf_like_domain/3'>Epidermal Growth Factor-like Domain</scene> is malleable and repositioning of this domain is essential for activation of the protein.<ref name="Reshetnyak" /> This domain undergoes conformational changes upon ligand binding and when in contact with the TNF-like domain.<ref name="Reshetnyak" /> The <scene name='90/904332/Tnf_egf_interface/3'>interface between the EGF-like and TNF-like domains</scene> consists of primarily hydrophobic residues, which enables their flexibility with regards to one another.<ref name="Reshetnyak" /> Hydrogen bonding occurs mostly between residues on the TNF-like domain.<ref name="Reshetnyak" /> There is only one hydrogen bond between the domains that is between residues Y734 on the TNF-like interface and Y984 on the EGF-interface.<ref name="Reshetnyak" /> Major motifs in the EGF-like domain are major and minor β-hairpins, which are stabilized by 3 conserved disulfide bridges. <ref name="Reshetnyak" /> |
''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ||
== Extracellular Domain Binding == | == Extracellular Domain Binding == | ||
| Line 29: | Line 29: | ||
''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ||
=== Binding Site === | === Binding Site === | ||
| - | This site doesn't start out surrounding the [https://en.wikipedia.org/wiki/Ligand_(biochemistry) ligand], instead the ligand binding initiates [https://en.wikipedia.org/wiki/Conformational_change conformational changes] across the protein. The ligands for ALK have highly positively charged faces that interact with the TNF-like region, the primary ligand-binding site on the extracellular region<ref name="Li" />. [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) Salt bridges] between the positively charged residues on the ligand and negatively charged residues on the receptor are stabilized by ligand binding. Three of these <scene name='90/904331/Salt_bridge_overview/1'>salt bridges</scene> occur between <scene name='90/904331/Salt_bridge_859_140/3'>E859 and R140</scene>, <scene name='90/904331/Salt_bridge_974_136/4'>E974 and R136</scene>, and <scene name='90/904331/Salt_bridge_978_123_133/3'>E978 with both R123 and R133</scene>. These strong ionic interactions also induce the conformational changes in the extracellular domain that induce the signaling pathway. <ref name="Reshetnyak" /> | + | The binding site is located on the TNF-like region. <ref name="DeMunck">PMID:34646012</ref> This site doesn't start out surrounding the [https://en.wikipedia.org/wiki/Ligand_(biochemistry) ligand], instead the ligand binding initiates [https://en.wikipedia.org/wiki/Conformational_change conformational changes] across the protein. The ligands for ALK have highly positively charged faces that interact with the TNF-like region, the primary ligand-binding site on the extracellular region<ref name="Li" />. [https://en.wikipedia.org/wiki/Salt_bridge_(protein_and_supramolecular) Salt bridges] between the positively charged residues on the ligand and negatively charged residues on the receptor are stabilized by ligand binding. Three of these <scene name='90/904331/Salt_bridge_overview/1'>salt bridges</scene> occur between <scene name='90/904331/Salt_bridge_859_140/3'>E859 and R140</scene>, <scene name='90/904331/Salt_bridge_974_136/4'>E974 and R136</scene>, and <scene name='90/904331/Salt_bridge_978_123_133/3'>E978 with both R123 and R133</scene>. These strong ionic interactions also induce the conformational changes in the extracellular domain that induce the signaling pathway. <ref name="Reshetnyak" /> |
''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ''To return to Structure of ALK with ALKAL2 bound scene click here: <scene name='90/904331/Alk_full/1'>ALK bound to ALKAL2</scene>'' | ||
=== Dimerization of ALK === | === Dimerization of ALK === | ||
Revision as of 03:29, 21 April 2022
| This Sandbox is Reserved from February 28 through September 1, 2022 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1700 through Sandbox Reserved 1729. |
To get started:
More help: Help:Editing |
Anaplastic Lymphoma Kinase Extracellular Region
| |||||||||||
References
- ↑ Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997 Jan 30;14(4):439-49. doi: 10.1038/sj.onc.1200849. PMID:9053841 doi:http://dx.doi.org/10.1038/sj.onc.1200849
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Huang H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int J Mol Sci. 2018 Nov 2;19(11). pii: ijms19113448. doi: 10.3390/ijms19113448. PMID:30400214 doi:http://dx.doi.org/10.3390/ijms19113448
- ↑ 3.0 3.1 3.2 Della Corte CM, Viscardi G, Di Liello R, Fasano M, Martinelli E, Troiani T, Ciardiello F, Morgillo F. Role and targeting of anaplastic lymphoma kinase in cancer. Mol Cancer. 2018 Feb 19;17(1):30. doi: 10.1186/s12943-018-0776-2. PMID:29455642 doi:http://dx.doi.org/10.1186/s12943-018-0776-2
- ↑ Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009 May 27;420(3):345-61. doi: 10.1042/BJ20090387. PMID:19459784 doi:http://dx.doi.org/10.1042/BJ20090387
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 Reshetnyak AV, Rossi P, Myasnikov AG, Sowaileh M, Mohanty J, Nourse A, Miller DJ, Lax I, Schlessinger J, Kalodimos CG. Mechanism for the activation of the anaplastic lymphoma kinase receptor. Nature. 2021 Dec;600(7887):153-157. doi: 10.1038/s41586-021-04140-8. Epub 2021, Nov 24. PMID:34819673 doi:http://dx.doi.org/10.1038/s41586-021-04140-8
- ↑ 6.0 6.1 6.2 6.3 Borenas M, Umapathy G, Lai WY, Lind DE, Witek B, Guan J, Mendoza-Garcia P, Masudi T, Claeys A, Chuang TP, El Wakil A, Arefin B, Fransson S, Koster J, Johansson M, Gaarder J, Van den Eynden J, Hallberg B, Palmer RH. ALK ligand ALKAL2 potentiates MYCN-driven neuroblastoma in the absence of ALK mutation. EMBO J. 2021 Feb 1;40(3):e105784. doi: 10.15252/embj.2020105784. Epub 2021 Jan 7. PMID:33411331 doi:http://dx.doi.org/10.15252/embj.2020105784
- ↑ 7.0 7.1 7.2 Chen S, Wang B, Fu X, Liang Y, Chai X, Ye Z, Li R, He Y, Kong G, Lian J, Li X, Chen T, Zhang X, Qiu X, Tang X, Zhou K, Lin B, Zeng J. ALKAL1 gene silencing prevents colorectal cancer progression via suppressing Sonic Hedgehog (SHH) signaling pathway. J Cancer. 2021 Jan 1;12(1):150-162. doi: 10.7150/jca.46447. eCollection 2021. PMID:33391411 doi:http://dx.doi.org/10.7150/jca.46447
- ↑ Reshetnyak AV, Murray PB, Shi X, Mo ES, Mohanty J, Tome F, Bai H, Gunel M, Lax I, Schlessinger J. Augmentor alpha and beta (FAM150) are ligands of the receptor tyrosine kinases ALK and LTK: Hierarchy and specificity of ligand-receptor interactions. Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):15862-7. doi:, 10.1073/pnas.1520099112. Epub 2015 Nov 16. PMID:26630010 doi:http://dx.doi.org/10.1073/pnas.1520099112
- ↑ De Munck S, Provost M, Kurikawa M, Omori I, Mukohyama J, Felix J, Bloch Y, Abdel-Wahab O, Bazan JF, Yoshimi A, Savvides SN. Structural basis of cytokine-mediated activation of ALK family receptors. Nature. 2021 Oct 13. pii: 10.1038/s41586-021-03959-5. doi:, 10.1038/s41586-021-03959-5. PMID:34646012 doi:http://dx.doi.org/10.1038/s41586-021-03959-5
- ↑ 10.0 10.1 10.2 10.3 Li T, Stayrook SE, Tsutsui Y, Zhang J, Wang Y, Li H, Proffitt A, Krimmer SG, Ahmed M, Belliveau O, Walker IX, Mudumbi KC, Suzuki Y, Lax I, Alvarado D, Lemmon MA, Schlessinger J, Klein DE. Structural basis for ligand reception by anaplastic lymphoma kinase. Nature. 2021 Dec;600(7887):148-152. doi: 10.1038/s41586-021-04141-7. Epub 2021, Nov 24. PMID:34819665 doi:http://dx.doi.org/10.1038/s41586-021-04141-7
- ↑ 11.0 11.1 Carpenter EL, Haglund EA, Mace EM, Deng D, Martinez D, Wood AC, Chow AK, Weiser DA, Belcastro LT, Winter C, Bresler SC, Vigny M, Mazot P, Asgharzadeh S, Seeger RC, Zhao H, Guo R, Christensen JG, Orange JS, Pawel BR, Lemmon MA, Mosse YP. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. 2012 Nov 15;31(46):4859-67. doi: 10.1038/onc.2011.647. Epub 2012 Jan, 23. PMID:22266870 doi:http://dx.doi.org/10.1038/onc.2011.647