Journal:Acta Cryst D:S2059798322009755
From Proteopedia
(Difference between revisions)

| Line 1: | Line 1: | ||
| - | <StructureSection load='' size='450' side='right' scene='93/931041/Cv1/1' caption=''> | + | α<StructureSection load='' size='450' side='right' scene='93/931041/Cv1/1' caption=''> |
===Structural and functional investigation of the human snRNP assembly factor AAR2 in complex with the PRPF8 RNaseH domain=== | ===Structural and functional investigation of the human snRNP assembly factor AAR2 in complex with the PRPF8 RNaseH domain=== | ||
<big>Marco Preussner, Karine F. Santos, Jonathan Alles, Christina Heroven, Florian Heyd, Markus C. Wahl, Gert Weber</big> <ref>doi: 10.1107/S2059798322009755</ref> | <big>Marco Preussner, Karine F. Santos, Jonathan Alles, Christina Heroven, Florian Heyd, Markus C. Wahl, Gert Weber</big> <ref>doi: 10.1107/S2059798322009755</ref> | ||
| Line 22: | Line 22: | ||
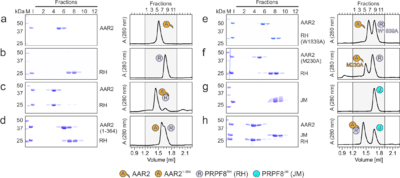
[[Image:Fig2AAR2.png|left|400px|thumb|Figure 2. Probing AAR2Δloop-PRPF8RH interacting regions and residues. (a-h) SDS-PAGE analyses (left) and UV elution profiles (right) of analytical size exclusion chromatography runs monitoring the interactions amongAAR2 variants, PRPF8RH 319 variants and PRPF8JM. Figures a-c were adapted from (Santos et al., 2015)<ref name='Santos'>PMID: 26527271</ref> and are shown for comparison. M, molecular mass standard (kDa); I, input samples. Protein bands are identified on the right. Elution fractions are indicated at the top of the gels and profiles, elution volumes are indicated at the bottom of the profiles. Icons are explained at the bottom. Variants are indicated at the respective icons. Peaks labeled by transparent icons represent an excess of the respective protein.]] | [[Image:Fig2AAR2.png|left|400px|thumb|Figure 2. Probing AAR2Δloop-PRPF8RH interacting regions and residues. (a-h) SDS-PAGE analyses (left) and UV elution profiles (right) of analytical size exclusion chromatography runs monitoring the interactions amongAAR2 variants, PRPF8RH 319 variants and PRPF8JM. Figures a-c were adapted from (Santos et al., 2015)<ref name='Santos'>PMID: 26527271</ref> and are shown for comparison. M, molecular mass standard (kDa); I, input samples. Protein bands are identified on the right. Elution fractions are indicated at the top of the gels and profiles, elution volumes are indicated at the bottom of the profiles. Icons are explained at the bottom. Variants are indicated at the respective icons. Peaks labeled by transparent icons represent an excess of the respective protein.]] | ||
{{Clear}} | {{Clear}} | ||
| + | |||
| + | <scene name='93/931041/Cv1/13'>Close-up view of the region in AAR2Δloop-PRPF8RH surrounding AAR2 Δloop S284</scene>. The corresponding region in yeast Aar2p is profoundly restructured upon replacement of the equivalent S253 by a phospho-mimetic glutamate residue <ref name='Weber'>PMID: 23442228</ref>. | ||
The structure and functional data of the human spliceosomal assembly factor Aar2 in complex with a core spliceosomal domain of the PRPF8 protein indicates a different function of human Aar2 in contrast to the yeast protein. | The structure and functional data of the human spliceosomal assembly factor Aar2 in complex with a core spliceosomal domain of the PRPF8 protein indicates a different function of human Aar2 in contrast to the yeast protein. | ||
Revision as of 13:56, 24 October 2022
α
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.